Abstract
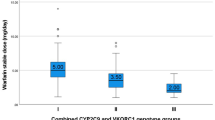
Warfarin is the most commonly used drug for chronic prevention of thromboembolic events, it also ranks high among drugs that cause serious adverse events. The variability in dose requirements has been attributed to inter-individual differences in medical, personal, and genetic factor. Cytochrome P-450 2C9 is the principle enzyme that terminates the anticoagulant effect of warfarin by catalyzing the conversion of the pharmacologically more potent S-enantiomer to its inactive metabolites. Warfarin exerts its effect by inhibition of vitamin K epoxide reductase. This protein is encoded by vitamin K epoxide reductase complex subunit 1 gene (VKORC1). The current study aimed to investigate the pharmacogenetic effect of CYP2C9 and VKORC1 gene polymorphisms on the patients response to warfarin. One hundred cases starting warfarin treatment and 20 healthy controls were enrolled. The mean daily dose of warfarin was calculated from patient’s medical records. For each patient, less than 10 % variability in warfarin dose and a target international normalized ratio (INR) within the therapeutic target range were required for at least 3 months for one of the following indications (deep vein thrombosis, pulmonary embolism, cerebrovascular stroke and myocardial infarction) prior to inclusion in the study. Tetraprimer amplification refractory mutation system PCR was performed to determine CYP2C9*2, CYP2C9*3, and the VKORC1 1639 G > A genetic polymorphisms. Plasma warfarin determination was performed using rapid fluorometric assay. Plasma warfarin concentration ranged from 2.19 to 10.98 μg/ml with a median 3.52 μg/ml. Supratherpeutic INR was observed in 11 % of the cases. Thromboembolic complications occurred in 7 % of the cases and 8 % of cases experienced major bleeding. High maintenance dose (>7 mg/day) was associated with the combined non VKORC1*2 and homozygous wild type CYP2C9 (CYP2C9*1*1) alleles, while low maintenance dose was associated with the Variant (AG + AA)/Wild (*1/*1). (p value <0.001). CYP2C9 variant was a risk factor for supratherapeutic INR in the multivariate logistic regression model. Thromboembolic complication and incidence of supratherapeutic INR were observed in patients carrying combined VKORC1 Variant (AG + AA) and CYP2C9 Variant (*2/*3). Data from our study suggest that together with clinical factors, VKORC1 and CYP2C9 polymorphisms are important contributors to warfarin dosing and may help predict adverse effects in Egyptian patients.


Similar content being viewed by others
Change history
19 March 2018
The aim of this erratum is to acknowledge that the original version of this article was inadvertently published with incorrect author name and affiliation for a co-author.
References
Wang L, McLeod HL, Weinshilboum RM (2011) Genomics and drug response. N Engl J Med 364(12):1144–1153
Lee AYY, Peterson EA (2013) Treatment of cancer-associated thrombosis. Blood 122(14):2310–2317
De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann F et al (2013) Vitamin K antagonists in heart disease: current status and perspectives (Section III). Thromb Haemost 110(6):1087–1107
Azuma K, Ouchi Y, Inoue S (2014) Vitamin K: novel molecular mechanisms of action and its roles in osteoporosis. Geriatr Gerontol Int 14(1):1–7
Falcone TD, Kim SS, Cortazzo MH (2011) Vitamin K: fracture prevention and beyond. PM&R 3(6):S82–S87
Bazan N, Sabry N, Rizk A, Mokhtar S, Badary O (2014) Factors affecting warfarin dose requirements and quality of anticoagulation in adult Egyptian patients: role of gene polymorphism. Ir J Med Sci 183(2):161–172
Jorgensen AL, FitzGerald RJ, Oyee J, Pirmohamed M, Williamson PR (2012) Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. PLoS ONE 7(8):e44064
Eckman MH, Rosand J, Greenberg SM, Gage BF (2009) Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med 150(2):73–83
Yin T, Miyata T (2007) Warfarin dose and the pharmacogenomics of <i> CYP2C9 </i> and <i> VKORC1 </i>—rationale and perspectives. Thromb Res 120(1):1–10
Wei M, Ye F, Xie D, Zhu Y, Zhu J, Tao Y et al (2012) A new algorithm to predict warfarin dose from polymorphisms of CYP4F2, CYP2C9 and VKORC1 and clinical variables: derivation in Han Chinese patients with non valvular atrial fibrillation. Thromb Haemost 107(6):1083
Lund K, Gaffney D, Spooner R, Etherington AM, Tansey P, Tait RC (2012) Polymorphisms in VKORC1 have more impact than CYP2C9 polymorphisms on early warfarin international normalized ratio control and bleeding rates. Br J Haematol 158(2):256–261
D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V et al (2005) A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 105(2):645–649
Wang D, Chen H, Momary KM, Cavallari LH, Johnson JA, Sadée W (2008) Regulatory polymorphism in vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood 112(4):1013–1021
Johnson J, Gong L, Whirl-Carrillo M, Gage B, Scott S, Stein C et al (2011) Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther 90(4):625–629
Miyagata Y, Nakai K, Sugiyama Y (2011) Clinical significance of combined CYP2C9 and VKORC1 genotypes in Japanese patients requiring warfarin. Int Heart J 52(1):44–49
Poe BL, Haverstick DM, Landers JP (2012) Warfarin genotyping in a single PCR reaction for microchip electrophoresis. Clin Chem 58(4):725–731
Corn M, Berberich R (1967) Rapid fluorometric assay for plasma warfarin. Clin Chem 13(2):126–131
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992
Yang J, Chen Y, Li X, Wei X, Chen X, Zhang L et al (2013) Influence of CYP2C9 and VKORC1 genotypes on the risk of hemorrhagic complications in warfarin-treated patients: a systematic review and meta-analysis. Int J Cardiol 168(4):4234–4243
El Din M, Amin D, Ragab S, Ashour E, Mohamed M, Mohamed A (2012) Frequency of VKORC1 (C1173T) and CYP2C9 genetic polymorphisms in Egyptians and their influence on warfarin maintenance dose: proposal for a new dosing regimen. Int J Lab Hematol 34(5):517–524
Gage BF, Lesko LJ (2008) Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis 25(1):45–51
Cho H-J, Sohn K-H, Park H-M, Lee K-H, Choi B, Kim S et al (2007) Factors affecting the interindividual variability of warfarin dose requirement in adult Korean patients. Pharmacogenomics 8(4):329–337
Namazi S, Azarpira N, Hendijani F, Khorshid MB, Vessal G, Mehdipour AR (2010) The impact of genetic polymorphisms and patient characteristics on warfarin dose requirements: a cross-sectional study in Iran. Clin Ther 32(6):1050–1060
Kimura R, Miyashita K, Kokubo Y, Akaiwa Y, Otsubo R, Nagatsuka K et al (2007) Genotypes of vitamin K epoxide reductase, γ-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb Res 120(2):181–186
Wells P, Majeed H, Kassem S, Langlois N, Gin B, Clermont J et al (2010) A regression model to predict warfarin dose from clinical variables and polymorphisms in CYP2C9, CYP4F2, and VKORC1: derivation in a sample with predominantly a history of venous thromboembolism. Thromb Res 125(6):e259–e264
Moreau C, Bajolle F, Siguret V, Lasne D, Golmard J-L, Elie C et al (2012) Vitamin K antagonists in children with heart disease: height and VKORC1 genotype are the main determinants of the warfarin dose requirement. Blood 119:861–867
Zohir N, Afifi R, Ahmed A, Aly Z, Elsobekey M, Kareem H et al (2013) Role of CYP2C9, VKORC1 and calumenin genotypes in monitoring warfarin therapy: an Egyptian study. Maced J Med Sci 6(4):414–420
Scordo MG, Pengo V, Spina E, Dahl ML, Gusella M, Padrini R (2002) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance[ast]. Clin Pharmacol Ther 72(6):702–710
Herman D, Locatelli I, Grabnar I, Peternel P, Stegnar M, Mrhar A et al (2005) Influence of CYP2C9 polymorphisms, demographic factors and concomitant drug therapy on warfarin metabolism and maintenance dose. Pharmacogenomics J 5(3):193–202
Lombardi R, Chantarangkul V, Cattaneo M, Tripodi A (2003) Measurement of warfarin in plasma by high performance liquid chromatography (HPLC) and its correlation with the international normalized ratio. Thromb Res 111(4):281–284
Krajciova L, Deziova L, Petrovic R, Luha J, Turcani P, Chandoga J (2013) Frequencies of polymorphisms in CYP2C9 and VKORC1 genes influencing warfarin metabolism in Slovak population: implication for clinical practice. Bratisl Lek Listy 115(9):563–568
Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A et al (2008) Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med 358(10):999–1008
Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM et al (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287(13):1690–1698
Ferder NS, Eby CS, Deych E, Harris JK, Ridker PM, Milligan PE et al (2010) Ability of VKORC1 and CYP2C9 to predict therapeutic warfarin dose during the initial weeks of therapy. J Thromb Haemost 8(1):95–100
Aithal GP, Day CP, Kesteven PJL, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353(9154):717–719
Ogg MS, Brennan P, Meade T, Humphries SE (1999) CYP2C9* 3 allelic variant and bleeding complications. Lancet 354(9184):1124
Limdi N, McGwin G, Goldstein J, Beasley T, Arnett D, Adler B et al (2008) Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 83(2):312–321
Roth JA, Boudreau D, Fujii MM, Farin FM, Rettie AE, Thummel KE et al (2014) Genetic risk factors for major bleeding in patients treated with warfarin in a community setting. Clin Pharmacol Ther 95(6):636–643
Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N et al (2009) A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 5(3):e1000433
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Ethical Standards
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Bedewy, A.M.L., Showeta, S., Mostafa, M.H. et al. The Influence of CYP2C9 and VKORC1 Gene Polymorphisms on the Response to Warfarin in Egyptians. Indian J Hematol Blood Transfus 34, 328–336 (2018). https://doi.org/10.1007/s12288-016-0725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-016-0725-4