Abstract
Background
Cyclin-dependent kinase (CDK) 4/6 inhibitors represent a significant advancement in the treatment of estrogen receptor (ER)-positive human epidermal growth factor receptor 2-negative advanced breast cancer. However, mechanisms of alterations after acquired resistance to CDK4/6 inhibitors and the optimal treatment options are still not established.
Methods
Abemaciclib-resistant cell lines were established from the models of estrogen deprivation-resistant cell lines which retained ER expression and activated ER function derived from MCF-7 breast cancer cell lines. Ribocilib-resistant cell lines were established in the same method as previously reported.
Results
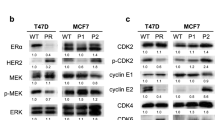
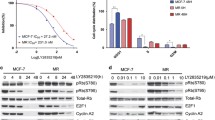
Both abemaciclib- and ribociclib-resistant cell lines showed decreased ER expression. ER transcriptional activity was maintained in these cell lines; however, the sensitivity to 4-hydroxytamoxifen and fulvestrant was almost completely lost. These cell lines did not exhibit any ERα gene mutation. Abemaciclib-resistant cell lines demonstrated low sensitivity to other CDK4/6 inhibitors; sensitivities to PI3K inhibitor, mTOR inhibitor, and chemotherapeutic drugs were maintained.
Conclusions
Dependence on ER signaling appears to decrease after the development of acquired resistance to CDK4/6 inhibitors. Further, CDK4/6 inhibitor-resistant cells acquired cross-resistance to other CDK4/6 inhibitors, PI3K/Akt/mTOR inhibitor therapy and chemotherapeutic drugs might serve as optimal treatment options for such breast cancers.





Similar content being viewed by others
References
Abubakar M, Sung H, Bcr D, Guida J, Tang TS, Pfeiffer RM, et al. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018;20:114.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol. 2016;17:425–39. https://doi.org/10.1016/S1470-2045(15)00613-0.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48. https://doi.org/10.1056/NEJMoa1609709.
Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–84.
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–36. https://doi.org/10.1056/NEJMoa1810527.
Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, erbb2-negative breast cancer that progressed on endocrine therapy—MONARCH 2. JAMA Oncol. 2019;94305:1–9.
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–16.
Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16:207.
Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18:1–11. https://doi.org/10.1186/s13058-015-0661-5.
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. https://doi.org/10.1186/bcr2419.
Iida M, Nakamura M, Tokuda E, Toyosawa D. The p21 levels have the potential to be a monitoring marker for ribociclib in breast cancer. Oncotarget. 2019;10:4907–18.
Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–64.
Fujiki N, Konno H, Kaneko Y, Gohno T, Hanamura T, Imami K, et al. Estrogen Response element-GFP (ERE-GFP) introduced MCF-7 cells demonstrated the coexistence of multiple estrogen-deprivation resistant mechanismsÏ. J Steroid Biochem Mol Biol. 2014;139:61–72. https://doi.org/10.1016/j.jsbmb.2013.08.012.
Tsuboi K, Kaneko Y, Nagatomo T, Fujii R, Hanamura T, Gohno T, et al. Different epigenetic mechanisms of ERα implicated in the fate of fulvestrant-resistant breast cancer. J Steroid Biochem Mol Biol. 2017;167:115–25. https://doi.org/10.1016/j.jsbmb.2016.11.017.
Hanamura T, Niwa T, Nishikawa S, Konno H, Gohno T, Tazawa C, et al. Androgen metabolite-dependent growth of hormone receptor-positive breast cancer as a possible aromatase inhibitor-resistance mechanism. Breast Cancer Res Treat. 2013;139:731–40.
Higuchi T, Endo M, Hanamura T, Gohno T. Contribution of estrone sulfate to cell proliferation in aromatase inhibitor (ai)-resistant, hormone receptor-positive breast cancer. PLoS ONE. 2016;11:e0155844.
Fujii R, Hanamura T, Suzuki T, Gohno T, Shibahara Y, Niwa T, et al. Increased androgen receptor activity and cell proliferation in aromatase inhibitor-resistant breast carcinoma. J Steroid Biochem Mol Biol. 2014;144:513–22. https://doi.org/10.1016/j.jsbmb.2014.08.019.
Hole S, Pedersen AM, Hansen SK, Lundqvist J, Yde CW, Lykkesfeldt AE. New cell culture model for aromatase inhibitor-resistant breast cancer shows sensitivity to fulvestrant treatment and cross-resistance between letrozole and exemestane. Int J Oncol. 2015;46:1481–90.
Perou CM, Sørile T, Eisen MB, Van De Rijn M, Jeffrey SS, Ress CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–766. https://doi.org/10.1016/j.ccr.2009.11.024.
Watts CKW, Sweeney KJE, Warlters A, Musgrove EA, Sutherland RL. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1994;31:95–105.
Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569:560–4. https://doi.org/10.1038/s41586-019-1056-z.
Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–67.
Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Tarazona N, Fenwick K, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015. https://doi.org/10.1126/scitranslmed.aac7551.
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–403. https://doi.org/10.1158/2159-8290.CD-18-0264.
Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene. 2010;29:4018–32.
Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–13.
Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29:640–5.
Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23:5561–72.
Raspé E, Coulonval K, Pita JM, Paternot S, Rothé F, Twyffels L, et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol Med. 2017;9:1052–66. https://doi.org/10.15252/emmm.201607084.
de Leeuw R, McNair C, Schiewer MJ, Neupane NP, Brand LJ, Augello MA, et al. MAPK reliance via acquired CDK4/6 inhibitor resistance in cancer. Clin Cancer Res. 2018;24:4201–14. https://doi.org/10.1158/1078-0432.CCR-18-0410.
O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–30. https://doi.org/10.1038/nrclinonc.2016.26.
Funding
This study was funded in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Science and Technology of Japan, a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan, the Program for Promotion of Fundamental Studies in Health Science of the National Institute of Biomedical Innovation (NIBIO), and a grant from the Smoking Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shin-ichi Hayashi received research grants from Novartis Pharma K.K and Astra Zeneca K.K.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iida, M., Toyosawa, D., Nakamura, M. et al. Decreased ER dependency after acquired resistance to CDK4/6 inhibitors. Breast Cancer 27, 963–972 (2020). https://doi.org/10.1007/s12282-020-01090-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01090-3