Abstract
Background
A lack of consistent methods to evaluate Ki67 expression is problematic in terms of accurately predicting prognosis in breast cancer. Accordingly, this study aimed to identify the causes of discrepancies in Ki67 labeling index measurements by different observers under different conditions using breast cancer samples.
Patients and methods
This Japanese study group compared and assessed immunohistochemical (IHC) analysis of the Ki67 labeling index when measured by different pathologists. Six pathologists (pathologists A–F) in Japan participated in this ring study. One hundred and ten surgical cases of estrogen receptor-positive and human epidermal growth factor receptor 2-negative invasive breast cancer treated in 2007 were identified from the breast cancer database of Tokai University Hospital and were included in this study.
Results
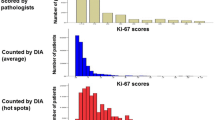
For all 6 pathologists, the Ki67 labeling index were significantly different between grade 3 and grade 1 cases and between grade 3 and grade 2 cases, whereas the index tended to be different between grade 1 and grade 2 cases. Further, the Ki67 labeling indexes measured by the 6 pathologists were strongly correlated (ρ: 0.73–0.88). The IHC scores recorded by pathologist A were in moderate to good agreement with those recorded by the others in patients with a Ki67 labeling index of <13.25 % and in those with a Ki67 labeling index of >13.25 % (κ = 0.429–0.660). The Ki67 low and high concordance rates between pathologist A and the others were 0.452–0.778 and 0.862–0.979, respectively. The most pertinent reason for discrepancy in scores seemed to be the selection of the area for counting and the quality of nuclear staining.
Conclusion
The Ki67 labeling index measured by 6 pathologists without method standardization was in fair to good agreement. We plan to undertake a second ring study, pending recommendations by the international Ki67 panel.






Similar content being viewed by others
References
Ohno S, Chow LW, Sato N, Masuda N, Sasano H, Takahashi F, et al. Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil-epirubicin-cyclophosphamide (FEC) in early-stage breast cancer: exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;142(1):69–80.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
Niikura N, Iwamoto T, Masuda S, Kumaki N, Xiaoyan T, Shirane M, et al. Immunohistochemical Ki67 labeling index has similar proliferation predictive power to various gene signatures in breast cancer. Cancer Sci. 2012;103:1508–12.
Urruticoechea A. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–20.
de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12155 patients. Br J Cancer. 2007;96:1504–13.
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health Recurrence Score in early breast cancer. J Clin Oncol. 2011;29(32):4273–8.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50.
Hugh J, Hanson J, Cheang MCU, Nielsen TO, Perou CM, Dumontet C, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–76.
von Minckwitz G, Schmitt WD, Loibl S, Muller BM, Blohmer JU, Sinn BV, et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2013;19:4521–31.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–23.
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. JNCI J Natl Cancer Inst. 2011;103(22):1656–64.
Jonat W, Arnold N. Is the Ki-67 labelling index ready for clinical use? Ann Oncol. 2011;22:500–2.
Barton S, Zabaglo L, A’Hern R, Turner N, Ferguson T, O’Neill S, et al. Assessment of the contribution of the IHC4 + C score to decision making in clinical practice in early breast cancer. Br J Cancer. 2012;106:1760–5.
Tsuda H, Akiyama F, Kurosumi M, Sakamoto G, Watanabe T. The efficacy and limitations of repeated slide conferences for improving interobserver agreement when judging nuclear atypia of breast cancer. The Japan National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) Pathology Section. Jpn J Clin Oncol. 1999;29:68–73.
Pinhel IF, Macneill FA, Hills MJ, Salter J, Detre S, A’Hern R, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res: BCR. 2010;12:R76.
Benini E, Rao S, Daidone MG, Pilotti S, Silvestrini R. Immunoreactivity to MIB-1 in breast cancer: methodological assessment and comparison with other proliferation indices. Cell Prolif. 1997;30:107–15.
Mengel M, von Wasielewski R, Wiese B, Rudiger T, Muller-Hermelink HK, Kreipe H. Inter-laboratory and inter-observer reproducibility of immunohistochemical assessment of the Ki-67 labelling index in a large multi-centre trial. J Pathol. 2002;198:292–9.
Mikami Y, Ueno T, Yoshimura K, Tsuda H, Kurosumi M, Masuda S, et al. Interobserver concordance of Ki67 labeling index in breast cancer: Japan Breast Cancer Research Group Ki67 Ring Study. Cancer Sci. 2013;104(11):1539–43.
Denkert C, Loibl S, Muller BM, Eidtmann H, Schmitt WD, Eiermann W, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2013;24(11):2786–93.
Hida AI, Oshiro Y, Inoue H, Kawaguchi H, Yamashita N, Moriya T. Visual assessment of Ki67 at a glance is an easy method to exclude many luminal-type breast cancers from counting 1000 cells. Breast Cancer. 2013. doi:10.1007/s12282-013-0460-8.
Tamaki K, Ishida T, Tamaki N, Kamada Y, Uehara K, Miyashita M, et al. Analysis of clinically relevant values of Ki-67 labeling index in Japanese breast cancer patients. Breast Cancer. 2012;21(3):325–33.
Viale G, Regan MM, Mastropasqua MG, Maffini F, Maiorano E, Colleoni M, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. JNCI J Natl Cancer Inst. 2008;100:207–12.
Williams DJ, Cohen C, Darrow M, Page AJ, Chastain B, Adams AL. Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol. 2011;19:431–6.
Acknowledgments
This work was supported by a research fund from the Japanese Breast Cancer Society.
Conflict of interest
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Niikura, N., Sakatani, T., Arima, N. et al. Assessment of the Ki67 labeling index: a Japanese validation ring study. Breast Cancer 23, 92–100 (2016). https://doi.org/10.1007/s12282-014-0536-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-014-0536-0