Abstract
Purpose of Review
This review discusses the connections between the gut-lung axis, gut and respiratory tract dysbiosis, and Candida bloodstream, oral, and respiratory infections in COVID-19 patients.
Recent Findings
COVID-19–related dysfunction in the intestinal barrier together with gut and lung dysbiosis played an important role in disease pathophysiology, which affected host immune homeostasis giving rise to prominent systemic and respiratory bacterial and fungal infections. Higher incidence of Candida bloodstream infections driven by accumulation of “classic” risk factors in severely ill COVID-19 patients was noted. Moreover, numerous C. auris outbreaks, characterized by high clonality of the strains, were reported from all around the world. Unlike other Candida species, C. auris colonization and infection cases most likely resulted from nosocomial transmission.
Summary
Infections due to Candida species in severely ill COVID-19 patients reflected the overall immune dysregulation and were largely driven by gut and respiratory tract dysbiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic has wreaked a devastating impact on global health with mortality approaching 7 million people [1]. People at the highest risk of severe COVID-19 were those of advanced age and those with comorbidities including hypertension, diabetes, chronic heart, and renal diseases [2]. Approximately 5–6% of symptomatic infected patients developed atypical pneumonia requiring hospitalization with many of them progressing to the intensive care unit (ICU) with respiratory failure and a further subset developing a more lethal cytokine storm resulting in an acute respiratory distress syndrome (ARDS) requiring mechanical ventilation [3]. A hallmark of severely ill COVID-19 patients was the development of a profound immune dysfunction [4] promoting the emergence of opportunistic bacterial, fungal, and viral infections [5,6,7,8]. Bacterial infections were often manifested as secondary pneumonias, urinary tract infections, and sepsis and were closely associated with prolonged hospitalization, mechanical ventilation, and the presence of invasive medical devices [9,10,11]. Individuals with latent tuberculosis infection were at increased risk of developing active tuberculosis due to the immune system’s compromised state caused by COVID-19 [5•]. The extensive use of antibiotics for treatment and prophylaxis, well known to disrupt the normal gut microbiota, increased the risk for developing Clostridium difficile infections within the gastrointestinal (GI) tract [12]. COVID-19 patients were also at heightened risk for developing viral infections due to herpes simplex virus (HSV), cytomegalovirus (CMV), and other respiratory co-infections [10, 13].
In recent years, it has been recognized that patients with certain severe viral and bacterial respiratory tract infections, including influenza, tuberculosis, and those with chronic diseases like chronic obstructive pulmonary disease (COPD), are prone to invasive fungal infections [6]. Seriously ill hospitalized patients with COVID-19 displayed an array of known risk factors for invasive fungal infections including lung damage resulting in a need for oxygen therapy, profound immunosuppression, and monoclonal antibody and corticosteroid therapy [14, 15•]. Such patients have impaired immune function of proinflammatory cytokines like interleukins IL-6, IL-1, IL-12, tumor necrosis factor (TNF), and interferon gamma (IFNγ), which promote opportunistic fungal infections [16]. Hence, patients with severe COVID-19 were also prone to develop invasive fungal infections [15•], particularly those caused by Candida [17••], Mucorales, and Aspergillus species [18] resulting in COVID-19–associated pulmonary aspergillosis (CAPA) [19, 20], COVID-19–associated mucormycosis (CAM) [21], and COVID-19–associated candidiasis (CAC) [22]. The high prevalence of CAC was not surprising given immune and barrier dysregulation in the gut and lung [22]. CAC carried a higher mortality than candidemia in non-COVID-19 patients during the same period [6, 23•]. It is the importance of gut-lung axis, gut, and respiratory tract dysbiosis and resulting bloodstream, oral and respiratory infections during COVID-19 that is discussed in this review.
Gut-Lung Axis in COVID-19
The lower gastrointestinal tract contains a complex microbiome of bacteria, fungi, and viruses, which are largely kept in-check in healthy individuals through host and microbial interactions [24]. The intestinal mucosa is a critical component that serves as a functional barrier. However, a breach in host containment can turn harmless commensal organisms into disease-causing pathogens that have life-threatening consequences for a patient resulting in sepsis, bloodstream infection, hyper inflammatory state, and multisystem failures [25]. The intestinal immune system harbors over 80% of the total body’s lymphocyte population residing in intraepithelial, lamina propria, Peyer’s patches, and mesenteric lymph nodes. Peyer’s patches and mesenteric lymph form aggregates with the latter connected to lymphatic system via drainage channels. The Peyer’s patches in concert with epithelial cells help induce local immune responses by mediating antigen presenting cell/T-cell interactions and release of cytokines [26]. Gut microbiota and their metabolites shape a healthy balance of Th17 and Treg cells [27]. Growing evidence supports strong crosstalk between the gut microbiota and lung, likely through the same interactions that maintain host health/disease balance [28], and the term “gut-lung axis” was created to describe this phenomenon.
During COVID-19, severely ill patients developed profound immune dysregulation and were often treated with broad-spectrum antibiotics and anti-inflammatory drugs, e.g., corticosteroids and cytokine antagonists. The resulting gut microbiome dysbiosis was associated with translocation of bacteria into the blood [29, 30]. The gut has been described as a main driver of critical illness [31], which induces dysfunction in the intestinal barrier and its hyperpermeability enabling luminal microbiota and metabolites to escape. Colonizing organisms can traverse the barrier either via a transcellular pathway involving epithelial cells or through a paracellular path involving tight junctions between adjacent epithelial cells. Impaired epithelial barrier function is often observed in inflammatory diseases, cancer, and transplantation and is impacted by factors such immune dysfunction and treatment with corticosteroid, cytokine antagonists, and antibiotics, as well as high fat diets [32, 33].
Fungi residing in the gastrointestinal tract (gut mycobiome) play important roles in host immune homeostasis, metabolism, and infection prevention [34, 35]. Fungal dysbiosis in the gut is associated with numerous diseases, including inflammatory bowel disease [36], colorectal cancer [37], and asthma [38, 39]. It is now apparent that there is a strong association between the gut and respiratory health, which surfaced prominently with COVID-19 [28, 38]. The gut-lung connection has been demonstrated in human and murine studies with some lung diseases influenced by gut microbiome changes and vice versa [39]. Thus, it is not surprising, given the ability of SARS-CoV-2 to replicate in both the respiratory and digestive tracts [40], that gut mycobiome in COVID-19 patients was a focus of several studies. Lv et al. compared the gut mycobiome of COVID-19- and H1N1-infected patients and healthy individuals. They discovered that in infected patients (in the comparison to healthy controls), the fungal burden in the gut was higher and that the relative abundances of some fungi with important functions were lower, but those of several opportunistic pathogenic fungi were higher [41]. Zuo et al. specifically identified Candida albicans, Candida auris, and Aspergillus flavus proportions to be increased in COVID-19 patients’ gut [42••].
Similarly, lower respiratory tract dysbiosis with a shift to Candida species colonization and a decreased fungal diversity was noted in COVID-19 patients [43, 44••]. These data corroborate the notion of SARS-CoV-2–triggered disruption of lung immune homeostasis, leading to overgrowth of pathogenic bacteria and fungi, and inflammation.
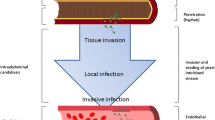
Altogether, as summarized in Fig. 1, COVID-19-related dysfunction in the intestinal barrier together with gut and lung dysbiosis played an important role in disease pathophysiology, which affected host immune homeostasis giving rise to prominent systemic and respiratory bacterial and fungal infections [30, 45, 46].
Candida Respiratory Tract Colonization and Candida Pneumonia in COVID-19 Patients
Candida spp. are frequently isolated from respiratory specimens, especially from ICU patients receiving mechanical ventilation [47,48,49]. It was estimated that up to 20% of patients acquired tracheobronchial colonization with Candida spp. after 48 h of intubation and ventilation and that their percent increases with extended ventilation [48]. However, an understanding of the significance of Candida spp. detection from respiratory samples is complicated, as it can represent one of the four scenarios: (1) contamination, an artifact introduced during sampling; (2) commensalism, member of the normal microbiome; (3) colonization, noninfectious resident that is not a member of a normal microbiome; and (4) infection, etiologic agent of infection. The diagnosis of Candida pneumonia should be confirmed by histopathology [47, 50]. Moreover, the presence of Candida spp. in any respiratory specimen always needs to be interpreted within its clinical and microbiological context, especially since there is a growing body of evidence of Candida spp. impact on human health even in noninfectious settings [51].
Candida pneumonia is rare, but colonization of the lower respiratory tract with Candida spp. has been associated with longer duration of mechanical ventilation, increased risk of ventilator-associated pneumonia (VAP), increased length of intensive care unit (ICU) and hospital stay, and higher mortality in mechanically ventilated patients [48, 52,53,54,55,56]. Major risk factors for Candida spp. acquisition in the respiratory tract include (1) host factors (STAT1 and dectin-1 defective mutations); (2) iatrogenic conditions (broad-spectrum antibiotics, mechanical ventilation, radiation therapy); (3) immunosuppression (neutropenia, systemic immunosuppression, steroid use, HIV, diabetes mellitus, bone marrow or solid organ transplant); and (4) extraneous (prolonged hospital stay, ICU stay, burns) [51].
Patients with severe viral respiratory tract infections are well recognized to be at high risk for developing invasive fungal infections including pulmonary aspergillosis and mucormycosis [13, 21]. Influenza pneumonias often present with increased disease morbidity and mortality, and similar disease co-dependence was observed during COVID-19 [14]. In a population of 100 immunosuppressed COVID-19 patients, Candida species were recovered from 69% of bronchoalveolar lavage specimens. Indeed, Candida colonization with restricted species reflected dysbiosis of lung and gut microbiota, which correlated with acute respiratory distress syndrome among patients [57]. Candida colonization in such severely ill patients is typically not deemed to directly impact clinical outcomes and is more a reflection of generalized immune, barrier and microbiota dysfunction [14]. Yet, its contribution to the overall state of COVID-19, including ARDS and other clinically significant risk factors, needs to be better assessed [58].
In a 2018–2022 study from France, both the incidence and prevalence of detection of Candida spp. in respiratory specimens increased in COVID-19 pandemic. Moreover, the length of stay in the hospital, mechanical ventilation, diabetes, and the use of antibacterials were identified as independent risk factors of Candida airway colonization [59••]. In Iran, C. albicans was found in the respiratory specimens of COVID-19 patients, especially those with diabetes, malignancies, and kidney disorders [57]. Similarly, we found virus- and drug-induced immunosuppression, together with prolonged hospital stay and mechanical ventilation, to increase the susceptibility to Candida colonization in the COVID-19 patients in New Jersey, USA [60]. Additionally, results of a Belgian study pointed to biofilms formed on endotracheal tubes (ETT), as a reservoir of microorganisms that can cause secondary infections in mechanically ventilated patients [43].
COVID-19–related epithelial damage of the airways gives way to fungal invasion in the respiratory tract. Although the most common agents of infection are molds of Aspergillus and Mucor genera, Candida lung infections, including C. albicans pneumonia with lung abscess [61], post-COVID-19 fungal empyema thoracis due to C. glabrata [62], and post-COVID-19 C. glabrata pneumonia [63], were presumptively reported, but histopathological evidence was provided only in one case [61].
Oral Candidiasis
The human commensal Candida albicans is a normal component of the oral cavity microbiota, and the development of oral and esophageal thrush is often a hallmark indication associated with immune dysfunction among patients with cancer and HIV/AIDS [64]. During COVID-19, the oral cavity was also impacted in patients resulting in typical oral clinical manifestations associated with systemic immune dysfunction including white and erythematous plaques, blisters, necrotizing gingivitis, ulcerations, salivary gland alterations, gustatory dysfunction, and coinfections [65]. Furthermore, overgrowth of Candida species was exacerbated by virus-infected salivary glands which compromised the production of histatin-5, a family of histidine-rich cationic antimicrobial proteins that help maintain a healthy balance of Candida in the oral biome [65]. Candida was frequently encountered in sputum samples, exceeding 53% in some studies, and due to the prolonged and chronic use of antifungal, high levels of mono- and multidrug resistance among Candida species isolates were reported [66].
Invasive Candida Infections in COVID-19 Patients
COVID-19–associated Candida spp. superinfections quickly became recognized as complications of the severe disease with the first four cases (C. albicans, n = 3; C. glabrata, n = 1) reported in 99 patients hospitalized in Wuhan Jinyintan Hospital (China) from Jan 1 to Jan 20, 2020 [67]. Further studies reported an increased incidence of Candida bloodstream infections (candidemia) in COVID-19 patients (in comparison to patients without COVID-19), especially in the ICU settings (Table 1). However, results of Candida spp. clinical isolates genotyping revealed that such an increase was not characterized by an uncontrolled nosocomial transmission [60, 68, 69•], except for the spread of Candida auris (see the next section).
Reasons for the higher frequency of candidemia in COVID-19 patients are still not fully understood. Unlike COVID-19–associated pulmonary aspergillosis (CAPA), where hyperinflammation is thought to be the main predisposing mechanism [15•], COVID-19–associated candidemia (CAC) most likely results from a combination of concomitant “classic” risk factors, such as prolonged hospital stay, ICU stay, (poorly controlled) diabetes mellitus, use of broad-spectrum antibiotics, use of corticosteroids, presence and duration of CVC, mechanical ventilation, and parenteral nutrition (Table 1). Also, as already discussed, a path to infection most likely resulted from dysbiosis of the fungal gut microbiome, decreased fungal diversity, and a shift toward Candida colonization in SARS-CoV-2–infected patients. Additionally, pandemic-related issues in overwhelmed healthcare facilities (crowded hospital rooms, decreased staff-to-patient ratios, limited availability of personal protective equipment (PPE)), leading to breaches in infection control practices (deviations from catheter management policies, inappropriate use of PPE), were possible contributors to the increased number of Candida infections in COVID-19 patients [50, 68, 77].
In most reports, the predominant identified species was C. albicans (Table 1), but some healthcare institutions noticed a trend of increasing non-albicans clinical isolates over the years. For example, in Gregorio Marañón Hospital in Madrid, Spain, the proportion of isolates between 2020 and 2022 decreased in C. albicans (60.3% vs. 36.7%) and increased in C. parapsilosis (10.3% vs. 28.6%) and C. tropicalis (8.8% vs. 16.3%) [69•]. Uniquely in India, C. auris was found to be the most predominant agent of CAC [96, 97].
Since the beginning of the COVID-19 pandemic, experts debated whether it would result in increased prevalence of antimicrobial resistance, with Clancy, Buehrle, and Nguyen saying “yes” and Collignon and Beggs saying “no.” However, they did not make any specific predictions regarding antifungal resistance [98,99,100]. Regrettably, antifungal drug susceptibility of the CAC isolates was determined rarely (Table 1), complicating comprehensive assessment of the situation and trend analysis. Posteraro et al. reported development of echinocandin resistance upon caspofungin treatment in a fatal case of COVID-19–associated C. glabrata infection [101].
Mortality in CAC patients was in the 28 to 100% range, with some healthcare institutions reporting significantly higher mortality in COVID‐19 patients than non‐COVID‐19 patients [17, 23, 95].
Candida auris in COVID-19 Patients
Even before the COVID-19 pandemic, Candida auris had already established itself as one of the hot topics among infectious diseases experts. In 2019, it was named an urgent threat in the CDC’s Antibiotic Resistance (AR) Threats Report due to its antifungal drug resistance and easy transmission, often leading to nosocomial outbreaks [102]. In the initial months of the pandemic, it was speculated that COVID-19 patients, especially the ones receiving critical care, would establish a population highly vulnerable to colonization and infection by C. auris [103]. These predictions proved correct, and numerous C. auris outbreaks occurred in countries all around the world (Table 2), as well as single cases in Japan [104], Qatar [105], and Turkey [106] were reported. Moreover, broader temporal analyses performed in India [79], Israel [107], and the USA [108, 109] informed of a growing number of C. auris cases during pandemic years. New C. auris introductions into previously unaffected healthcare facilities were also described [107, 110, 111]. The pooled mortality rate of C. auris candidiasis from published studies was estimated to exceed 60% (64.7% [112], 67.849% [113]).
The outbreaks were characterized by high clonality of the strains [107, 110, 114, 115, 118,119,120, 125, 127, 132] supporting the notion of intrahospital transmission of C. auris. Prolonged hospital stays, high burden of severely sick patients, and challenges in the implementation of infection control practices (e.g., extended or incorrect use of personal protective equipment) during the COVID-19 pandemic are thought to be the main drivers of patients’ colonization with C. auris [96, 112, 127]. Lengthy lockdowns and travel restrictions most likely also contributed to the local spread pattern [114, 127].
Following the CDC guidance [134], echinocandins were used as first-line therapy in invasive C. auris cases [96, 97, 107, 110, 112, 114, 115, 118, 125, 126, 131], followed by amphotericin B [96, 112, 118, 125, 126, 131] and azoles [107, 110, 118, 125, 126]. Antifungal drug susceptibility (if determined) was clade-dependent with isolates of clade I (South Asian) showing almost uniform fluconazole resistance and high rates of amphotericin B resistance (Table 2). However, in Brazil, the researchers found unexpected low antifungal minimal inhibitory concentration (MIC) values and the absence of any resistance-conferring mutations in clade I isolates [114, 115]. Only a few studies identified molecular determinants of antifungal drug resistance in recovered C. auris clinical isolates. Well-known azole resistance-conferring mutations included Erg11 Y132F and K143R from India [97], Erg11 K143R and Tac1b A640V from Italy (120), Erg11 Y132F from Lebanon [123] and Qatar [127], and Erg11 V125A/F126L from the USA [131]. Moreover, previously reported echinocandin resistance-conferring Fks1 mutations S639F and S639Y were detected Qatari isolates [127].
COVID-19 patients who developed C. auris infection were often severely ill with the most prevalent comorbidities being hypertension, diabetes mellitus, and cardiovascular diseases [96, 97, 113, 114, 121, 122, 124, 125, 130, 131, 133]. Other risk factors, including mechanical ventilation, extensive antibiotic use, steroid treatment, and placement of indwelling devices, also contributed to the C. auris infection acquisition [79, 96, 97, 107, 111, 114, 115, 117, 118, 121, 122, 124,125,126, 131,132,133]. In some cases, C. auris infection occurred concurrently with bacterial superinfection, further complicating patient management [107, 114, 118, 120, 124,125,126, 129, 133].
Public health professionals have speculated on the role of COVID-19 pandemic-related logistical issues, including low PPE compliance due to anticipated/existing PPE shortages and relaxation of the measures to control C. auris due to the higher workload of healthcare workers, which would promote nosocomial transmission of C. auris. Recent experience has highlighted the urgent need for uninterrupted C. auris surveillance and containment efforts.
Conclusion
COVID-19 patients who progressed to severe disease with acute respiratory distress were notable for their associated immune dysfunction and increased risk for developing opportunistic invasive fungal infections, including the ones caused by Candida species. Additionally, many of severely ill COVID-19 patients were treated with broad‐spectrum antibiotics disrupting the normal intestinal flora composition [135] and corticosteroids enhancing Candida cells adhesion to the epithelial cells [136]. The resulting dysbiosis with promotion of Candida growth in the gastrointestinal and respiratory tracts with eventual translocation of Candida to the bloodstream system led to an increased number of Candida infections in COVID-19 patients. For commensal organisms like C. albicans and C. glabrata, which form a prominent reservoir in the gut, COVID-19 highlighted the importance of the gut-lung axis. While Candida in respiratory fluids of patients with pneumonia was associated with high mortality, it did not rise to the level of attributable mortality. Yet, such organisms almost certainly increased the body’s overall inflammatory state contributing to patient decline. Early and appropriate management of lung and gut dysbiosis should become a part of routine standard-of-care for such patients with the aim of preventing the progression toward invasive Candida infections.
Finally, the steady rise of C. auris colonization and infection cases among hospitalized COVID-19 patients is a cautionary tale, as this environmentally hearty and drug-resistant organism continues to prey on the chronically ill immunocompromised hosts. Active surveillance of patient body sites (axilla, groin, nares) and healthcare environment is critical for limiting transmission and preventing infections. Here, molecular diagnostics methods offer rapid and accurate detection of patient and surface colonization and can aid in implementation of infection prevention and control measures especially in case of patient transfers.
Change history
13 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12281-023-00478-w
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. WHO Coronavirus (COVID-19) Dashboard: World Health Organization; 2023 [updated 07/19/2023. Available from: https://covid19.who.int/. Accessed 07/24/2023
CDC. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals: the Centers for Disease Control and Prevention; 2023 [updated 02/09/2023. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 07/24/2023
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–7.
• Abdoli A, Falahi S, Kenarkoohi A. COVID-19-associated opportunistic infections: a snapshot on the current reports. Clin Exp Med. 2022;22(3):327–46. Review providing a landscape of opportunistic infections in COVID-19.
Hlaing KM, Monday LM, Nucci M, Nouer SA, Revankar SG. Invasive fungal infections associated with COVID-19. J Fungi (Basel). 2023;9(6):667.
Altinkaya Cavus M, Sav H. Opportunistic Candida infections in critical COVID-19 patients. Pol J Microbiol. 2022;71(3):411–9.
Kangabam N, Nethravathy V. An overview of opportunistic fungal infections associated with COVID-19. 3 Biotech. 2023;13(7):231.
Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319.
Guchhait P, Chaudhuri BN, Das S. Bloodstream infections with opportunistic pathogens in COVID-19 era: a real challenge necessitates stringent infection control. J Lab Physicians. 2023;15(1):131–8.
Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–9.
Linares-Garcia L, Cardenas-Barragan ME, Hernandez-Ceballos W, Perez-Solano CS, Morales-Guzman AS, Miller DS, et al. Bacterial and fungal gut dysbiosis and Clostridium difficile in COVID-19: a review. J Clin Gastroenterol. 2022;56(4):285–98.
Kurra N, Woodard PI, Gandrakota N, Gandhi H, Polisetty SR, Ang SP, et al. Opportunistic infections in COVID-19: a systematic review and meta-analysis. Cureus. 2022;14(3):e23687.
Salazar F, Bignell E, Brown GD, Cook PC, Warris A. Pathogenesis of respiratory viral and fungal coinfections. Clin Microbiol Rev. 2022;35(1):e0009421.
• Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, Goldman GH, et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7(8):1127–40. Comprehensive overview of CAPA, CAM, and CAC, with highly informative timelines and maps.
Moser D, Biere K, Han B, Hoerl M, Schelling G, Chouker A, et al. COVID-19 impairs immune response to Candida albicans. Front Immunol. 2021;12:640644.
•• Seagle EE, Jackson BR, Lockhart SR, Georgacopoulos O, Nunnally NS, Roland J, et al. The landscape of candidemia during the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin Infect Dis. 2022;74(5):802–11. Report on the epidemiology of candidemia in the USA during the early months of the COVID-19 pandemic with comparison to two non–COVID-19 cohorts.
Yusuf E, Vonk A, van den Akker JPC, Bode L, Sips GJ, Rijnders BJA, de Steenwinkel J, Verkaik NJ, Vogel M, van der Eerden M, van Westreenen M. Frequency of Positive Aspergillus Tests in COVID-19 Patients in Comparison to Other Patients with Pulmonary Infections Admitted to the Intensive Care Unit. J Clin Microbiol. 2021;59(3):e02278-20. https://doi.org/10.1128/JCM.02278-20.
Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, Cornely OA, S Perlin D, Lass-Flörl C, Hoenigl M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)-From Immunology to Treatment. J Fungi (Basel). 2020;6(2):91. https://doi.org/10.3390/jof6020091
Dimopoulos G, Almyroudi MP, Myrianthefs P, Rello J. COVID-19-associated pulmonary aspergillosis (CAPA). J Intensive Med. 2021;1(2):71–80.
Krishna V, Bansal N, Morjaria J, Kaul S. COVID-19-Associated Pulmonary Mucormycosis. J Fungi (Basel). 2022;8(7):711. https://doi.org/10.3390/jof8070711.
Tsai CS, Lee SS, Chen WC, Tseng CH, Lee NY, Chen PL, Li MC, Syue LS, Lo CL, Ko WC, Hung YP. COVID-19-associated candidiasis and the emerging concern of Candida auris infections. J Microbiol Immunol Infect. 2023;56(4):672–9. https://doi.org/10.1016/j.jmii.2022.12.002.
• Kayaaslan B, Eser F, Kaya Kalem A, Bilgic Z, Asilturk D, Hasanoglu I, et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64(9):1083–91. Comprehensive study providing information on Candida species distribution and antifungal drug susceptibilities in COVID-19 and non-COVID-19 patients.
Liang D, Leung RK, Guan W, Au WW. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018;10:3.
Konig J, Wells J, Cani PD, Garcia-Rodenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10):e196.
Cabrera-Perez J, Badovinac VP, Griffith TS. Enteric immunity, the gut microbiome, and sepsis: rethinking the germ theory of disease. Exp Biol Med (Maywood). 2017;242(2):127–39.
Cheng H, Guan X, Chen D, Ma W. The Th17/Treg cell balance: a gut microbiota-modulated story. Microorganisms. 2019;7(12):583.
Aktas B, Aslim B. Gut-lung axis and dysbiosis in COVID-19. Turk J Biol. 2020;44(3):265–72.
Venzon M, Bernard-Raichon L, Klein J, Axelrad JE, Zhang C, Hussey GA, Sullivan AP, Casanovas-Massana A, Noval MG, Valero-Jimenez AM, Gago J, Putzel G, Pironti A, Wilder E, Yale IMPACT Research Team, Thorpe LE, Littman DR, Dittmann M, Stapleford KA, Shopsin B, Torres VJ, Ko AI, Iwasaki A, Cadwell K, Schluter J. Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation. Nat Commun. 2022;13(1):5926. https://doi.org/10.1101/2021.07.15.452246
Bernard-Raichon L, Venzon M, Klein J, Axelrad JE, Zhang C, Sullivan AP, et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat Commun. 2022;13(1):5926.
Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23(2):143–8.
Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–23.
Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. 2019;7:17.
Kong HH, Segre JA. Cultivating fungal research. Science. 2020;368(6489):365–6.
Chin VK, Yong VC, Chong PP, Amin Nordin S, Basir R, Abdullah M. Mycobiome in the gut: a multiperspective review. Mediators Inflamm. 2020;2020:9560684.
Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–48.
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019;68(4):654–62.
Kanj AN, Kottom TJ, Schaefbauer KJ, Choudhury M, Limper AH, Skalski JH. Dysbiosis of the intestinal fungal microbiota increases lung resident group 2 innate lymphoid cells and is associated with enhanced asthma severity in mice and humans. Respir Res. 2023;24(1):144.
Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52(2):241–55.
Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis. 2021;73(3):361–6.
Lv L, Gu S, Jiang H, Yan R, Chen Y, Chen Y, et al. Gut mycobiota alterations in patients with COVID-19 and H1N1 infections and their associations with clinical features. Commun Biol. 2021;4(1):480.
•• Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159(4):1302-10 e5. Study revealing that COVID-19 patients had significant alterations in their fecal mycobiomes (enrichment in Candia albicans) compared with controls.
van Charante F, Wieme A, Rigole P, De Canck E, Ostyn L, Grassi L, et al. Microbial diversity and antimicrobial susceptibility in endotracheal tube biofilms recovered from mechanically ventilated COVID-19 patients. Biofilm. 2022;4:100079.
•• Xie L, Chen L, Li X, Zhou J, Tian H, Zhao J, et al. Analysis of lung microbiome in COVID-19 patients during time of hospitalization. Pathogens. 2023;12(7):944. Study exploring bacterial and fungal microbiota in the lung of COVID-19 patients in comparison to non-COVID-19 pneumonia controls.
Prasad R, Patton MJ, Floyd JL, Fortmann S, DuPont M, Harbour A, et al. Plasma microbiome in COVID-19 subjects: an indicator of gut barrier defects and dysbiosis. Int J Mol Sci. 2022;23(16):9141.
Sun Z, Song ZG, Liu C, Tan S, Lin S, Zhu J, et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022;20(1):24.
Liu J, Yu YT, Xu CH, Chen DC. Candida colonization in the respiratory tract: what is the significance? Front Med (Lausanne). 2020;7:598037.
Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR, et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest. 2006;129(1):110–7.
Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274(8):639–44.
Peman J, Ruiz-Gaitan A, Garcia-Vidal C, Salavert M, Ramirez P, Puchades F, et al. Fungal co-infection in COVID-19 patients: should we be concerned? Rev Iberoam Micol. 2020;37(2):41–6.
Pendleton KM, Huffnagle GB, Dickson RP. The significance of Candida in the human respiratory tract: our evolving understanding. Pathog Dis. 2017;75(3):ftx029. https://doi.org/10.1093/femspd/ftx029.
Delisle MS, Williamson DR, Perreault MM, Albert M, Jiang X, Heyland DK. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care. 2008;23(1):11–7.
Hamet M, Pavon A, Dalle F, Pechinot A, Prin S, Quenot JP, et al. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med. 2012;38(8):1272–9.
Safdar A, Armstrong D. Prospective evaluation of Candida species colonization in hospitalized cancer patients: impact on short-term survival in recipients of marrow transplantation and patients with hematological malignancies. Bone Marrow Transplant. 2002;30(12):931–5.
Huang D, Qi M, Hu Y, Yu M, Liang Z. The impact of Candida spp airway colonization on clinical outcomes in patients with ventilator-associated pneumonia: a systematic review and meta-analysis. Am J Infect Control. 2020;48(6):695–701.
Pendleton KM, Dickson RP, Newton DW, Hoffman TC, Yanik GA, Huffnagle GB. Respiratory tract colonization by Candida species portends worse outcomes in immunocompromised patients. Clin Pulm Med. 2018;25(6):197–201.
Erami M, Raiesi O, Momen-Heravi M, Getso MI, Fakhrehi M, Mehri N, et al. Clinical impact of Candida respiratory tract colonization and acute lung infections in critically ill patients with COVID-19 pneumonia. Microb Pathog. 2022;166:105520.
McCarty T. Candidemia and severe Coronavirus Disease 2019: which risk factors are modifiable? Clin Infect Dis. 2022;74(5):812–3.
•• Froidefond M, Sevestre J, Chaudet H, Ranque S. COVID-19 is a confounder of increased candida airway colonisation. Pathogens. 2023;12(3):463. The authors report increased incidence and prevalence of Candida-positive respiratory samples in COVID-19 patients and associate Candida colonisation in the respiratory tract with 7x increased risk for developing invasive fungal disease.
Kordalewska M, Guerrero KD, Garcia-Rubio R, Jimenez-Ortigosa C, Mediavilla JR, Cunningham MH, et al. Antifungal drug susceptibility and genetic characterization of fungi recovered from COVID-19 patients. J Fungi (Basel). 2021;7(7):552.
Abdelhadi A, Kassem A. Candida pneumonia with lung abscess as a complication of severe COVID-19 pneumonia. Int Med Case Rep J. 2021;14:853–61.
Swaminathan N, Anderson K, Nosanchuk JD, Akiyama MJ. Candida glabrata empyema thoracis-a post-COVID-19 complication. J Fungi (Basel). 2022;8(9):923.
Nathania E, Widjaja J. Candida glabrata pneumonia in post COVID-19 patient: a rare case report. Am J Respir Crit Care Med. 2022;205:A4569.
Cernakova L, Rodrigues CF. Microbial interactions and immunity response in oral Candida species. Future Microbiol. 2020;15:1653–77.
Naqvi AR, Schwartz J, Brandini DA, Schaller S, Hussein H, Valverde A, et al. COVID-19 and oral diseases: assessing manifestations of a new pathogen in oral infections. Int Rev Immunol. 2022;41(4):423–37.
Cut TG, Mavrea A, Cumpanas AA, Novacescu D, Oancea CI, Bratosin F, et al. A retrospective assessment of sputum samples and antimicrobial resistance in COVID-19 patients. Pathogens. 2023;12(4):620.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Machado M, Estevez A, Sanchez-Carrillo C, Guinea J, Escribano P, Alonso R, et al. Incidence of candidemia is higher in COVID-19 versus non-COVID-19 patients, but not driven by intrahospital transmission. J Fungi (Basel). 2022;8(3):305.
•Díaz-García J, Mesquida A, Machado M, Sánchez-Carrillo C, Muñoz P, Escribano P, Guinea J. Yeasts from blood cultures in the wake of the COVID-19 pandemic in a tertiary care hospital: Shift in species epidemiology, steady low antifungal resistance and full in vitro ibrexafungerp activity. Med Mycol. 2023;61(7):myad072. https://doi.org/10.1093/mmy/myad072. Analysis of Candida species distribution throughout the pandemic years.
Seitz T, Holbik J, Grieb A, Karolyi M, Hind J, Gibas G, et al. The role of bacterial and fungal superinfection in critical COVID-19. Viruses. 2022;14(12):2785.
Nucci M, Barreiros G, Guimaraes LF, Deriquehem VAS, Castineiras AC, Nouer SA. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64(2):152–6.
Martins AC, Psaltikidis EM, de Lima TC, Fagnani R, Schreiber AZ, Conterno LO, et al. COVID-19 and invasive fungal coinfections: a case series at a Brazilian referral hospital. J Mycol Med. 2021;31(4):101175.
Riche CVW, Cassol R, Pasqualotto AC. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J Fungi (Basel). 2020;6(4):286.
Ramadan HK, Mahmoud MA, Aburahma MZ, Elkhawaga AA, El-Mokhtar MA, Sayed IM, et al. Predictors of severity and co-infection resistance profile in COVID-19 patients: first report from Upper Egypt. Infect Drug Resist. 2020;13:3409–22.
Negm EM, Mohamed MS, Rabie RA, Fouad WS, Beniamen A, Mosallem A, et al. Fungal infection profile in critically ill COVID-19 patients: a prospective study at a large teaching hospital in a middle-income country. BMC Infect Dis. 2023;23(1):246.
Blaize M, Raoelina A, Kornblum D, Kamus L, Lampros A, Berger M, et al. Occurrence of candidemia in patients with COVID-19 admitted to five ICUs in France. J Fungi (Basel). 2022;8(7):678.
Papadimitriou-Olivgeris M, Kolonitsiou F, Kefala S, Spiliopoulou A, Aretha D, Bartzavali C, et al. Increased incidence of candidemia in critically ill patients during the Coronavirus Disease 2019 (COVID-19) pandemic. Braz J Infect Dis. 2022;26(2):102353.
Szabo BG, Lakatos B, Bobek I, Szabo E, Szlavik J, Valyi-Nagy I. Invasive fungal infections among critically ill adult COVID-19 patients: first experiences from the national centre in Hungary. J Mycol Med. 2021;31(4):101198.
Rajni E, Jain A, Gupta S, Jangid Y, Vohra R. Risk Factors for candidemia in intensive care unit: a matched case control study from North-Western India. Acta Medica (Hradec Kralove). 2022;65(3):83–8.
Yazdanpanah S, Ahmadi M, Zare Z, Nikoupour H, Arabsheybani S, Jabrodini A, et al. Assessment of risk factors and clinical outcomes in hospitalized COVID-19 patients with Candida spp. co-infections: species distribution and antifungal susceptibility patterns of isolates. Mycopathologia. 2023;188(1–2):9–20.
Calderaro A, Buttrini M, Montecchini S, Piccolo G, Martinelli M, Dell’Anna ML, et al. Detection of SARS-CoV-2 and other infectious agents in lower respiratory tract samples belonging to patients admitted to intensive care units of a tertiary-care hospital, located in an epidemic area, during the Italian lockdown. Microorganisms. 2021;9(1):185.
Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564.
•• Mastrangelo A, Germinario BN, Ferrante M, Frangi C, Li Voti R, Muccini C, et al. Candidemia in Coronavirus Disease 2019 (COVID-19) patients: incidence and characteristics in a prospective cohort compared with historical non-COVID-19 controls. Clin Infect Dis. 2021;73(9):e2838–9. Well controlled study of candidemia among COVID-19 patient and historical non-COVID-19 controls.
Cataldo MA, Tetaj N, Selleri M, Marchioni L, Capone A, Caraffa E, et al. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming “collateral effect.” J Glob Antimicrob Resist. 2020;23:290–1.
Brikman S, Dori G, Kasher C, Yanovskay A, Strauss M, Colodner R, et al. Candida bloodstream infection, a dire complication in hospitalized COVID-19 patients: three cases from a single center in Northern Israel. Isr Med Assoc J. 2021;23(10):615–7.
Roman-Montes CM, Bojorges-Aguilar S, Corral-Herrera EA, Rangel-Cordero A, Diaz-Lomeli P, Cervantes-Sanchez A, et al. Fungal infections in the ICU during the COVID-19 pandemic in Mexico. J Fungi (Basel). 2023;9(5):583.
• Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F. COVID-19 associated invasive candidiasis. J Infect. 2021;82(2):e45–6. Review providing an overview of invasive fungal infections and highlighting the importance of invasive candidiasis among COVID-19 patients.
Agrifoglio A, Cachafeiro L, Figueira JC, Anon JM, Garcia de Lorenzo A. Critically ill patients with COVID-19 and candidaemia: we must keep this in mind. J Mycol Med. 2020;30(4):101012.
Segrelles-Calvo G, de S Araújo GR, Llopis-Pastor E, Carrillo J, Hernandez-Hernandez M, Rey L, et al. Candida spp. co-infection in COVID-19 patients with severe pneumonia: prevalence study and associated risk factors. Respir Med. 2021;188:106619.
Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–8.
White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose Coronavirus Disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021;73(7):e1634–44.
Macauley P, Epelbaum O. Epidemiology and mycology of candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021;64(6):634–40.
Bishburg E, Okoh A, Nagarakanti SR, Lindner M, Migliore C, Patel P. Fungemia in COVID-19 ICU patients, a single medical center experience. J Med Virol. 2021;93(5):2810–4.
Nori P, Cowman K, Chen V, Bartash R, Szymczak W, Madaline T, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42(1):84–8.
Bauer KA, Yu K, Moise PA, Finelli L, Ai C, Watts J, et al. Morbidity and mortality of hospitalised patients with candidemia during the various severe acute respiratory syndrome coronavirus 2 pandemic waves: a multicentre evaluation of 248 US hospitals. Mycoses. 2023;66(6):483–7.
Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis. 2020;26(11):2694–6.
Rajni E, Singh A, Tarai B, Jain K, Shankar R, Pawar K, et al. A high frequency of Candida auris blood stream infections in Coronavirus Disease 2019 patients admitted to intensive care units, Northwestern India: a case control study. Open Forum Infect Dis. 2021;8(12):ofab452.
Clancy CJ, Buehrle DJ, Nguyen MH. PRO: the COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob Resist. 2020;2(3):dlaa049.
Collignon P, Beggs JJ. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC Antimicrob Resist. 2020;2(3):dlaa051.
van Duin D, Barlow G, Nathwani D. The impact of the COVID-19 pandemic on antimicrobial resistance: a debate. JAC Antimicrob Resist. 2020;2(3):dlaa053.
Posteraro B, Torelli R, Vella A, Leone PM, De Angelis G, De Carolis E, et al. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. J Fungi (Basel). 2020;6(3):163.
CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019.
Chowdhary A, Sharma A. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J Glob Antimicrob Resist. 2020;22:175–6.
Ohashi Y, Matono T, Suzuki S, Yoshino S, Alshahni MM, Komori A, et al. The first case of clade I Candida auris candidemia in a patient with COVID-19 in Japan. J Infect Chemother. 2023;29(7):713–7.
Goravey W, Ali GA, Ali M, Ibrahim EB, Al Maslamani M, Abdel HH. Ominous combination: COVID-19 disease and Candida auris fungemia-case report and review of the literature. Clin Case Rep. 2021;9(9):e04827.
Bolukbasi Y, Erkose Genc G, Orhun G, Kuskucu MA, Cagatay A, Onel M, et al. First case of COVID-19 positive Candida auris fungemia in Turkey. Mikrobiyol Bul. 2021;55(4):648–55.
Biran R, Cohen R, Finn T, Brosh-Nissimov T, Rahav G, Yahav D, et al. Nationwide outbreak of Candida auris infections driven by COVID-19 hospitalizations, Israel, 2021–2022. Emerg Infect Dis. 2023;29(7):1297–301.
•• Lyman M, Forsberg K, Sexton DJ, Chow NA, Lockhart SR, Jackson BR, et al. Worsening spread of Candida auris in the United States, 2019 to 2021. Ann Intern Med. 2023;176(4):489–95. US CDC report of concerning rise in echinocandin-resistant Candida auris cases and evidence of its transmission.
Benedict K, Forsberg K, Gold JAW, Baggs J, Lyman M. Candida auris-associated hospitalizations, United States, 2017–2022. Emerg Infect Dis. 2023;29(7):1485–7.
Katsiari M, Mavroidi A, Kesesidis N, Palla E, Zourla K, Ntorlis K, et al. Emergence of clonally-related South Asian clade I clinical isolates of Candida auris in a Greek COVID-19 intensive care unit. J Fungi (Basel). 2023;9(2):243.
Allaw F, Kara Zahreddine N, Ibrahim A, Tannous J, Taleb H, Bizri AR, et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. 2021;10(2):157.
Vinayagamoorthy K, Pentapati KC, Prakash H. Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: a systematic review. Mycoses. 2022;65(6):613–24.
Vaseghi N, Sharifisooraki J, Khodadadi H, Nami S, Safari F, Ahangarkani F, et al. Global prevalence and subgroup analyses of coronavirus disease (COVID-19) associated Candida auris infections (CACa): a systematic review and meta-analysis. Mycoses. 2022;65(7):683–703.
de Almeida JN, Jr Francisco EC, Hagen F, Brandão IB, Pereira FM, Presta Dias PH, de Miranda Costa MM, de Souza Jordão RT, de Groot T, Colombo AL. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J Fungi (Basel). 2021;7(3):220. https://doi.org/10.3390/jof7030220.
Nobrega de Almeida J, Jr., Brandao IB, Francisco EC, de Almeida SLR, de Oliveira Dias P, Pereira FM, et al. 2021 Axillary digital thermometers uplifted a multidrug-susceptible Candida auris outbreak among COVID-19 patients in Brazil. Mycoses. 64(9):1062–72.
Escandon P, Caceres DH, Lizarazo D, Lockhart SR, Lyman M, Duarte C. Laboratory-based surveillance of Candida auris in Colombia, 2016–2020. Mycoses. 2022;65(2):222–5.
Rodriguez JY, Le Pape P, Lopez O, Esquea K, Labiosa AL, Alvarez-Moreno C. Candida auris: a latent threat to critically ill patients with Coronavirus Disease 2019. Clin Infect Dis. 2021;73(9):e2836–7.
• Hinrichs C, Wiese-Posselt M, Graf B, Geffers C, Weikert B, Enghard P, et al. Successful control of Candida auris transmission in a German COVID-19 intensive care unit. Mycoses. 2022;65(6):643–9. Authors report effective implementation of infection prevention and control measures to prevent transmission of Candida auris.
Di Pilato V, Codda G, Ball L, Giacobbe DR, Willison E, Mikulska M, et al. Molecular epidemiological investigation of a nosocomial cluster of C. auris: evidence of recent emergence in Italy and ease of transmission during the COVID-19 pandemic. J Fungi (Basel). 2021;7(2):140.
Magnasco L, Mikulska M, Giacobbe DR, Taramasso L, Vena A, Dentone C, et al. Spread of carbapenem-resistant Gram-negatives and Candida auris during the COVID-19 pandemic in critically ill patients: one step back in antimicrobial stewardship? Microorganisms. 2021;9(1):95.
Briano F, Magnasco L, Sepulcri C, Dettori S, Dentone C, Mikulska M, et al. Candida auris candidemia in critically ill, colonized patients: cumulative incidence and risk factors. Infect Dis Ther. 2022;11(3):1149–60.
Corcione S, Montrucchio G, Shbaklo N, De Benedetto I, Sales G, Cedrone M, et al. First cases of Candida auris in a referral intensive care unit in Piedmont Region, Italy. Microorganisms. 2022;10(8):1521.
Reslan L, Araj GF, Finianos M, El Asmar R, Hrabak J, Dbaibo G, et al. Molecular characterization of Candida auris isolates at a major tertiary care center in Lebanon. Front Microbiol. 2021;12:770635.
Allaw F, Haddad SF, Habib N, Moukarzel P, Naji NS, Kanafani ZA, et al. COVID-19 and C. auris: a case-control study from a tertiary care center in Lebanon. Microorganisms. 2022;10(5):1011.
Villanueva-Lozano H, Trevino-Rangel RJ, Gonzalez GM, Ramirez-Elizondo MT, Lara-Medrano R, Aleman-Bocanegra MC, et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect. 2021;27(5):813–6.
Moin S, Farooqi J, Rattani S, Nasir N, Zaka S, Jabeen K. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med Mycol. 2021;59(12):1238–42.
Ben Abid F, Salah H, Sundararaju S, Dalil L, Abdelwahab AH, Salameh S, Ibrahim EB, Almaslmani MA, Tang P, Perez-Lopez A, Tsui CKM. Molecular characterization of Candida auris outbreak isolates in Qatar from patients with COVID-19 reveals the emergence of isolates resistant to three classes of antifungal drugs. Clin Microbiol Infect. 2023;29(8):1083.e1–1083.e7. https://doi.org/10.1016/j.cmi.2023.04.025.
MuletBayona JV, TormoPalop N, Salvador Garcia C, FusterEscriva B, ChanzaAvino M, Ortega Garcia P, et al. Impact of the SARS-CoV-2 pandemic in candidaemia, invasive aspergillosis and antifungal consumption in a tertiary hospital. J Fungi (Basel). 2021;7(6):440.
Alfonso-Sanchez JL, Agurto-Ramirez A, Chong-Valbuena MA, De-Jesus-Maria I, Julian-Paches P, Lopez-Cerrillo L, et al. The influence of infection and colonization on outcomes in inpatients with COVID-19: are we forgetting something? Front Public Health. 2021;9:747791.
Senok A, Alfaresi M, Khansaheb H, Nassar R, Hachim M, Al Suwaidi H, et al. Coinfections in patients hospitalized with COVID-19: a descriptive study from the United Arab Emirates. Infect Drug Resist. 2021;14:2289–96.
de St Maurice A, Parti U, Anikst VE, Harper T, Mirasol R, Dayo AJ, Garner OB, Prabaker KK, Yang S. Clinical, microbiological, and genomic characteristics of clade-III Candida auris colonization and infection in southern California, 2019–2022. Infect Control Hosp Epidemiol. 2022;44(7):1–9. https://doi.org/10.1017/ice.2022.204.
Hanson BM, Dinh AQ, Tran TT, Arenas S, Pronty D, Gershengorn HB, et al. Candida auris invasive infections during a COVID-19 case surge. Antimicrob Agents Chemother. 2021;65(10):e0114621.
Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris outbreak in a COVID-19 specialty care unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):56–7.
CDC. Treatment and management of C. auris infections and colonization: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED); 2022 [updated 12/14/2022]. Available from: https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html. Accessed 07/12/2023
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912.
Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–38.
Funding
This work was supported by NIH grant R01AI109025 to D.S.P.
Author information
Authors and Affiliations
Contributions
M.K. and D.S.P. wrote the manuscript text; M.K. prepared figures and tables.
Corresponding authors
Ethics declarations
Competing interests
David S. Perlin receives funding from the U.S. National Institutes of Health (NIH) and contracts with Merck, Regeneron, and Pfizer. He serves on the advisory board for N8 Medical and holds patents on detection assays for fungi and their antifungal drug resistance. Milena Kordalewska declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Several text were found incorrect. Full information regarding the corrections made can be found in the erratum/correction for this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kordalewska, M., Perlin, D.S. Candida in COVID-19: Gut-Lung Axis, Dysbiosis, and Infections. Curr Fungal Infect Rep 17, 263–280 (2023). https://doi.org/10.1007/s12281-023-00476-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-023-00476-y