Abstract
Purpose
Secondary bacterial or fungal infections are one of the most important medical complications among patients with Coronavirus Disease 2019 (COVID-19). The emergence of multidrug-resistant (MDR) candida can cause many problems such as treatment failure, adverse clinical outcomes, and even disease outbreaks. This systematic review and meta-analysis aims to investigate the prevalence and outcomes of fungal drug-resistant in COVID-19 patients.
Methods
PubMed, Embase, Scopus, Cochrane Library, and Web of Science databases were searched for peer reviewed-articles published in English up to May 20, 2021. Heterogeneity across studies was evaluated using Cochrane’s Q test and the I2 index. The pooled point prevalence and their corresponding 95% confidence intervals (CIs) were considered to estimate the prevalence of fungal drug resistance infection in COVID-19 patients.
Results
Eight eligible articles were included in our meta-analysis. The number of COVID-19 patients with fungal co-infection varied from 5 to 35 among selected studies. The overall pooled prevalence of fungal drug resistance among patients with co-infections of fungal and COVID-19 was 69% (95% CI: 37%, 94%) by using a random-effects model. In terms of specific species, the pooled meta-analysis for Candida Auris was estimated to be 100% (95%CI: 98%, 100%; I2 = 0%), for Multi-Candida 59% (95%CI: 38%, 79%; I2 = 12.5%), and for Aspergillus 15% (95%CI: 0%, 42%; I2 = 0%).
Conclusion
Our study shows the high prevalence of fungal drug resistance in COVID-19 patients and emphasizes the need to strengthen antimicrobial stewardship programs, close monitoring for treatment failure, and the emergence of resistance upon treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important medical complications among patients with Coronavirus Disease 2019 (COVID-19) is secondary bacterial or fungal infections [1,2,3]. The frequent intakes of corticosteroids and antibiotics, receiving mechanical ventilation, and central venous catheter (CVC) use are major risk factors for secondary infections [4]. Comparing the situations between the pre-and post-COVID-19 era shows a greater deal of candidemia [5, 6]. Also, Aspergillus fumigatus has been identified as the leading cause of fungal infections in critically ill COVID-19 patients [7]. Fungal co-infections in COVID-19 patients have a higher incidence of acute infections and an increased mortality rate up to 83% despite anti-fungal treatment [8]. The emergence of multidrug-resistant (MDR) candida can cause serious problems such as treatment failure, adverse clinical outcomes, and even disease outbreaks. A study from Italy reported six patients admitted to the COVID-19 intensive care units (ICUs) infected with Candida Auris (C. Auris). All strains C. Auris identified proved to be resistant to amphotericin-B and azoles. Among patients with candidemia, they reported a 50% mortality rate after 25 days from first C. Auris isolation [9••]. Similarly, a recent study from Lebanon showed that among seven patients who had prior COVID-19, all the isolates were resistant to fluconazole and amphotericin B [10••]. Moreover, a recent study from Iran reported seven critically ill patients with COVID-19 who had fungemia, among whom six had candidemia. In this study, none of the isolates of Candida Glabrata (C. Glabrata) were drug-resistant. In contrast half of the patients infected with Candida albicans (C. Albicans) were resistant to both azoles and echinocandins. They were treated with fluconazole and caspofungin, which ultimately showed therapeutic failure, and the mortality rate due to C. Albicans and C. Glabrata was 100% [11••]. In conclusion, the global prevalence rate of fungal drug resistance in COVID-19 patients remains elusive. In our opinion, a possible underestimation of the risk of drug resistance may occur at the bedside of ICU patients with COVID-19. This needs to be estimated at a global level. This systematic review and meta-analysis investigated the prevalence and outcomes of fungal drug-resistant in COVID-19 patients.
Methods
Search strategy and selection criteria
With the help of a health sciences librarian (ZK), the electronic searches were performed in PubMed/MEDLINE, Embase, Scopus, Web of Science (ISI), and Cochrane Library for peer reviewed-articles published in English up to May 20, 2021. A combination of MeSH terms including: "COVID-19", "Coronavirus Disease 2019", "Novel Coronavirus", "Severe Acute Respiratory Syndrome Coronavirus 2", "SARS CoV 2 Infection", "Drug Resistance", "Antifungal Drug Resistance", "Multidrug Resistance", "MDR Resistance", "Fungus", "Candida Infection", "Fungal Infection", "Pulmonary Aspergillosis", "Mucormycose", "Histoplasma Capsulatum" were used (Suppl. 1). Additional searches were conducted according to Google Scholar and the reference lists of selected articles for more accuracy.
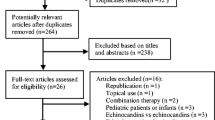
After screening the titles and abstracts and the full paper of the selected records were independently evaluated by two investigators (AH and M.A-K). A complete description of step by step of our search strategy is available in Fig. 1. The current study was conducted and reported according to PRISMA guidelines with PROSPERO registration number CRD42021260172.
Studies selection
Studies were included if were: 1) among patients with COVID-19 in all ages with confirmed respiratory syndrome coronavirus (SARS-CoV-2) infection and fungal co-infection, (2) reported sufficient data on fungal drug resistance, and 3) observational studies publishing in peer-reviewed journals. We excluded literature reviews, systematic reviews, case reports, letters to editors, non-English articles, studies concerning cell biology, and studies not investigating fungal drug resistance.
Data extraction
Two independent investigators (AH and M.A-K) extracted the data from each eligible study using customized data extraction forms in Excel spreadsheets. Disagreements were resolved with a provision for arbitration from a third reviewer (RT). The following data were extracted: first author name, year of publication, geographical region of the study, study type, number of COVID-19 patients, number of co-infections with fungal, number of cases with drug resistance of fungal organisms, main demographic characteristics of patients, ICU length of stay, basic associated-comorbidities among participants, steroid intake, and outcome at the end of the study.
Quality assessment
The Joanna Briggs Institute (JBI) Critical Appraisal guidelines were used to assess the quality of included studies [12]. Each article was evaluated using the 8-point JBI critical appraisal tool. This tool applies the following criteria to quality assessment: 1) Clearly stated inclusion/exclusion criteria, 2) Confirmation of the disease using a standard/reliable method for all participants, 3) Consecutive inclusion of participants, 4) Clear reporting of demographics in the study, 5) Clear reporting of clinical information of the participants (comorbidities), 6) Clear reporting of the site(s)/clinic(s) demographic information, 7) Clear reporting of the case outcomes or follow-ups, 8) Appropriate use of the statistical tests to assess the relevant outcomes. According to these dimensions, each study was assigned a score that is computed using different parameters in line with the review objectives. The responses received a score of 0 for “Not reported” or “No” and 1 for “Yes”. JBI critical appraisal tool score ranges from 0 to 8. The details of the quality assessment are presented in Supplementary Table 1.
Study synthesis
Relevant statistical analyses were performed using STATA version 12.0 (Stata Corp., College Station, TX). The pooled point prevalence and their corresponding 95% confidence intervals (CIs) were considered to estimate the prevalence rate of fungal drug resistance infection in COVID-19 patients. The Cochrane’s Q test and the I2 index were used to assess heterogeneity across studies. I2 > 50% with a P < 0.1 for Cochrane’s Q test indicated substantial heterogeneity. Due to a wide variation in fungal drug resistance reported by included studies, ranging from 0 to 100 percent, we combined the point prevalence using the metaprop function with the Freeman-Tukey double arcsine transformation method. A random-effects model was applied for pooling all of the point prevalence. Furthermore, subgroup analyses were conducted to explore the source of heterogeneity based on moderator variables including the species of fungal organisms (C. Auris vs. Multi-Candida vs. Aspergillus), studying the effect of the region (European vs. Asian vs. other). A sensitivity analysis was performed to indicate the reliability of pooled results with user-specified I2 (25%). Another sensitivity analysis was performed after excluding low-quality studies.
Results
Search findings
Our initial searches from electronic databases found a total of 1639 records. Of these, 373 duplicate citations were excluded using EndNote software. After screening titles and abstracts, 1094 irrelevant records were removed, and 172 full papers of the remaining articles were retrieved to assess according to our inclusion criteria. Finally, eight eligible articles were selected for the current meta-analysis.
All eight included studies were peer-reviewed observational studies [9••, 10••, 11••, 13,14,15,16,17]. The number of COVID-19 patients with total fungal co-infection varied from 5 to 35 among included studies. The age range of the patients was 43 to 72 years old [10••, 13]. Of these, one article was considered drug resistance on Aspergillus co-infection (this study reported two patient populations; patients from the first wave of COVID-19 from March until May 1, 2020, and patients from the second wave from September to December 2020.) [15] and the remaining seven articles on different species of Candida [9••, 10••, 11••, 13, 14, 16, 17]. Three out of eight included articles were carried out in the Netherlands [15] and Italy [9••, 17], and the remaining studies were performed in United States (Florida) [14], Egypt [13], Lebanon [10••], India [16], and Iran [11••]. The demographic characteristics of the articles are presented in Table 1.
Main outcomes
The point prevalence for each study and pooled prevalence of fungal drug resistance in hospitalized fungi and COVID-19 patients are shown in Fig. 2.
The prevalence of fungal drug resistance across included studies varied from 13% in the study performed by Meijer et al., in the Netherlands [15] to 100% in studies conducted by Allaw et al., in Lebanon [10••] and Prestel et al., in the United States (Florida) [14].
Based on eight selected articles, the overall pooled prevalence of fungal drug resistance among patients with co-infections of fungal and COVID-19 was 69% (95% CI: 37%, 94%) by using a random-effects model.
Substantial heterogeneity was identified among studies (I2 = 86.60%, P < 0.01). Result of subgroup analysis showed significant decreases in heterogeneity based on the species of fungal organisms (C. Auris vs. Multi-Candida vs. Aspergillus). In terms of specific species, the pooled meta-analysis for C. Auris was estimated to be 100% (95%CI: 98%, 100%; I2 = 0%), for Multi-Candida 59% (95%CI: 38%, 79%; I2 = 12.5%), and for Aspergillus 15% (95%CI: 0%, 42%; I2 = 0%).
In a subgroup analysis to study the effect of the region, the pooled meta-analysis for European was estimated to be 41% (95%CI: 1%, 89%; I2 = 81.4%), for Asian 81% (95%CI: 54%, 99%; I2 = 53.35%), and for others (America and Africa) 100% (95%CI: 94%, 100%; I2 = 0%).
In a sensitivity analysis when we indicated the validity of overall pooled results using user-specified I2 (25%), the overall pooled estimation of fungal drug resistance among patients with co-infections of fungal and COVID-19 did not significantly change: 69% (95% CI: 37%, 94%) was before and 68% (95% CI: 35%, 94%) after this analysis. In another sensitivity analysis when we excluded two low-quality studies [18], the overall pooled estimation of fungal drug resistance among patients with co-infections of fungal and COVID-19 did not significantly change: 69% (95% CI: 37%, 94%) was before and 66% (95% CI: 36%, 91%) after the analysis.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis that investigating the prevalence of fungal drug resistance in patients hospitalized with COVID-19. Meta-analysis using a random-effects model demonstrated a significant prevalence of fungal drug resistance, both overall and within subgroup analyses.
Before the emergence of the COVID-19 pandemic, drug resistance was considered a major global health threat due to high rates of hospitalization and death. [19] It is estimated that the number of deaths due to infections with multiple drug-resistant (MDR) pathogens will reach 10 million per year by 2050 [20].
Different bacterial, viral, and fungal infections may complicate COVID-19 symptoms especially in critically ill patients admitted to ICU. It is declared that the prevalence of superinfection at ICU admission was 21.7% and over half of the cases catch at least one infection during their ICU stay [21]. Risk factors associated with COVID-19 and fungemia, are mostly a consequence of the severity of the disease including; mechanical ventilation, prolonged hospital or ICU stays, excessive corticosteroid use, and multiple antibiotics intakes [22]. Moreover, other factors such as advanced age and underlying systemic diseases also contribute to fungemia [23].
In line with previous reports, our results reflect the global nature of this pandemic. The pooled prevalence of fungal drug resistance was 69% but varied slightly by species, ranging from 15% for Aspergillus to 100% for C. Auris. Subgroup analyses based on the species of fungal organisms including C. Auris vs. Multi-Candida vs. Aspergillus. C. Auris showed a 100% prevalence, while 59% for Multi-Candida and 15% for Aspergillus. Potential explanations for this difference include differences in the studied patient populations, regarding disease severity and setting. For example, one study included in this review involved both moderate and severe cases of COVID-19 [13] while others included SARS-CoV-2 PCR-positive patients who needed intubation and mechanical ventilation or ICU admitted patients [9••, 11••, 15,16,17]. Furthermore, only one included article reported Aspergillosis [15] and others demonstrated different species of Candida including C. Auris, C. Albicans, and C. Glabrata. The discordance in the prevalence of the aforementioned fungi also appears to be consistent with prior evidence documenting the MDR characteristics of C. Auris and the difficulties of eradicating it [24].
COVID-19 causes prolonged stay of critically ill patients in the ICU. Invasive candidiasis (IC) is an infection that can be caused by several Candida species, presents as a spectrum of disease: from minimally symptomatic candidaemia to fulminant sepsis [25]. Additionally IC is the most common fungal infection among patients admitted to the ICU [26]. Additionally, long-term hospitalization of the patients in critical units is the main risk factor for acquiring IC [27]. The development of acute respiratory distress syndrome (ARDS) in these cases predisposes them to different secondary bacterial and fungal infections [28]. A recent study by Moser et al. demonstrated that COVID-19 infection impairs immunity response and prone patients with ARDS in the ICU to Candida Albicans infection [29]. Besides, the epidemiology of IC has evolved during recent years with an increasing incidence of non-Albicans Candida species noted globally [30]. These species including C. Glabrata and C. Auris show intrinsic and/or acquired resistance to antifungals which adversely affect the successful treatment of the organism [28]. Hopefully, the development of new antifungal agents for treating C. Auris infections such as ibrexafungerp and rezafungin provides new insights into the management of these MDR yeasts [31].
Aspergillus species can cause co-infections in patients with severe COVID-19 or those admitted to ICU with or without tracheal intubation [7]. The reported incidence of invasive pulmonary aspergillosis in COVID-19 patients varies from 19.6% to 33.3% [32, 33]. Both voriconazole and isavuconazole are recommended as the first-line treatments for aspergillosis, though azole-resistant strains are a new concern in COVID-19 patients. For whom, polyene antifungal treatment such as amphotericin B is suggested with a favorable outcome [34].
Antimicrobial resistance has become one of the most serious global issues. And Asia is one of the epicenters of antimicrobial drug resistance, there is a growing concern about disseminating MDR pathogens [35, 36]. Therefore, we conducted a subanalysis based on studies regions including European vs. Asian vs. other. Asia showed an 81% prevalence, and 41% for Europe. Our results were in line with previous studies and it's most likely due to poor global health infrastructure in most Asian countries.
In terms of underlying conditions [37], we were unable to further subanalysis the included studies due to unavailable information. We did not have data about patients whether had certain underlying conditions like malignancy, diabetes mellitus, or renal failure. Further studies investigating the association between fungal drug resistance and comorbidities, particularly in the context of COVID-19 infection, would be useful to assess.
Taken together, our results show that the emergence of MDR to any drug class severely eliminate treatment options which have in turn led to high mortality and poor outcome among COVID-19 patients. Therefore, the antimicrobial stewardship program must be strengthened for patients with SARS-CoV-2 infection; close monitoring for treatment failure and the emergence of resistance upon treatment is needed to ensure rapid case identification, appropriate treatment, and coordination with infection prevention to minimize transmission [38, 39].
Our systematic review and meta-analysis have several strengths. We followed a comprehensive literature search strategy with the help of a health sciences librarian and we used a dual-reviewer process to screen and select relevant studies meeting the inclusion criteria. However, the present study has some limitations that should be acknowledged. Firstly, only eight studies were reviewed, and the relatively small overall sample had limited power to further explore these relationships. Secondly, the heterogeneity of the included studies was another limitation, though we performed a subgroup analysis to address this limitation. Accordingly, we believe more comprehensive studies are required.
Conclusion
In summary, our study found that among patients with COVID-19, the overall prevalence of fungal drug resistance is high, with approximately 100% prevalence for C. Auris drug resistance. Raising awareness of this fact may enhance standard care in patients with co-infections of fungal and COVID-19.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–9.
Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–68.
Peng J, Wang Q, Mei H, Zheng H, Liang G, She X, et al. Fungal co-infection in COVID-19 patients: evidence from a systematic review and meta-analysis. Aging (Albany NY). 2021;13(6):7745.
Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76(4):1078–84.
Nucci M, Barreiros G, Guimarães LF, Deriquehem VAS, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64(2):152–6.
Mastrangelo A, Germinario BN, Ferrante M, Frangi C, Li Voti R, Muccini C, et al. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID-19 Controls. Clin Infect Dis. 2020:ciaa1594.
Lai C-C, Yu W-L. COVID-19 associated with pulmonary aspergillosis: A literature review. J Microbiol Immunol Infect. 2021;54(1):46–53.
Villanueva-Lozano H, Treviño-Rangel RdJ, González GM, Ramírez-Elizondo MT, Lara-Medrano R, Aleman-Bocanegra MC, et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect. 2021;27(5):813–6.
•• Magnasco L, Mikulska M, Giacobbe DR, Taramasso L, Vena A, Dentone C, et al. Spread of carbapenem-resistant gram-negatives and candida auris during the covid-19 pandemic in critically ill patients: One step back in antimicrobial stewardship. Microorg. 2021;9(1):95. (This study reported six patients admitted to the COVID-19 ICUs infected with C. Auris. All strains C. Auris identified proved to be resistant to amphotericin-B and azoles and highlighted the importance of further studies to determine the impact of COVID-19 antimicrobial stewardship.••)
•• Allaw F, Kara Zahreddine N, Ibrahim A, Tannous J, Taleb H, Bizri AR, et al. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens. 2021;10(2). This study showed that among seven patients who had prior COVID-19, all the isolates were resistant to fluconazole and amphotericin B. And highlights the emerging threat of C. Auris in the COVID-19 pandemic. ••
•• Arastehfar A, Shaban T, Zarrinfar H, Roudbary M, Ghazanfari M, Hedayati M-T, et al. Candidemia among Iranian Patients with Severe COVID-19 Admitted to ICUs. J Fungi (Basel). 2021;7(4):280. (This study reported seven critically ill patients with COVID-19 who had fungemia, among whom six had candidemia. Half of the patients infected with C. Albicans were resistant to both azoles and echinocandins. They were treated with fluconazole and caspofungin, which ultimately showed therapeutic failure, and the mortality rate due to C. Albicans and C. Glabrata was 100%. The high mortality rate of patients, despite antifungal therapy, demonstrated the importance of rapid diagnosis of fungal infections among COVID-19 patients.)
Aromataris E, Munn Z. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute, 2017. 2020.
Ramadan HK, Mahmoud MA, Aburahma MZ, Elkhawaga AA, El-Mokhtar MA, Sayed IM, et al. Predictors of Severity and Co-Infection Resistance Profile in COVID-19 Patients: First Report from Upper Egypt. Infect Drug Resist. 2020;13:3409–22.
Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):56.
Meijer EFJ, Dofferhoff ASM, Hoiting O, Meis JF. COVID-19–associated pulmonary aspergillosis: a prospective single-center dual case series. Mycoses. 2021;64(4):457–64.
Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April-July 2020. Emerg Infect Dis. 2020;26(11):2694–6.
Cataldo MA, Tetaj N, Selleri M, Marchioni L, Capone A, Caraffa E, et al. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming “collateral effect.” J Glob Antimicrob Resist. 2020;23:290–1.
Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. Morb Mortal Wkly Rep. 2021;70(2):56.
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–18.
Grande-Bretagne RoAR. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations: December 2014: Review on Antimicrobial Resistance; 2014.
Signorini L, Moioli G, Calza S, Van Hauwermeiren E, Lorenzotti S, Del Fabro G, et al. Epidemiological and Clinical Characterization of Superinfections in Critically Ill Coronavirus Disease 2019 Patients. Critical care explorations. 2021;3(6): e0430.
Bishburg E, Okoh A, Nagarakanti SR, Lindner M, Migliore C, Patel P. Fungemia in COVID-19 ICU Patients, a Single Medical Center Experience. J Med Virol. 2020;93(5):2810–4.
Seagle EE, Jackson BR, Lockhart SR, Georgacopoulos O, Nunnally NS, Roland J, et al. The landscape of candidemia during the COVID-19 pandemic. Clin Infect Dis. 2021:ciab562.
Osei SJ. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen. 2018;7(4):e00578.
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026.
Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent JL. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Critical care (London, England). 2016;20(1):125.
Zhang Z, Zhu R, Luan Z, Ma X. Risk of invasive candidiasis with prolonged duration of ICU stay: a systematic review and meta-analysis. BMJ Open. 2020;10(7):e036452.
Arastehfar A, Carvalho A, Nguyen MH, Hedayati MT, Netea MG, Perlin DS, et al. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J Fungi (Basel). 2020;6(4).
Moser D, Biere K, Han B, Hoerl M, Schelling G, Choukér A, et al. COVID-19 Impairs Immune Response to Candida albicans. Front Immunol. 2021;12:640644.
Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73(suppl_1):i4-i13.
Giacobbe DR, Magnasco L, Sepulcri C, Mikulska M, Koehler P, Cornely OA, et al. Recent advances and future perspectives in the pharmacological treatment of Candida auris infections. Expert Rev Clin Pharmacol. 2021.
Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8(6):e48–9.
van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated Pulmonary Aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132–5.
Koehler P, Bassetti M, Chakrabarti A, Chen SC, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–62.
Kang C-I, Song J-H. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infection & chemotherapy. 2013;45(1):22–31.
Yam ELY, Hsu LY, Yap EP-H, Yeo TW, Lee V, Schlundt J, et al. Antimicrobial Resistance in the Asia Pacific region: a meeting report. Springer; 2019.
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5.
Perfect JR, Ghannoum M. Emerging Issues in Antifungal Resistance. Infect Dis Clin North Am. 2020;34(4):921–43.
Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921.
Acknowledgements
This study was conducted at the Noncommunicable Diseases Research Center of Fasa University of Medical Sciences, Fars, Iran, with a grant number: IR.FUMS.REC.1400.056.
Funding
No financial assistance was received in support of the study.
Author information
Authors and Affiliations
Contributions
RT and MA contributed to conception. The databases were searched by ZK and AH and M.A-K contributed significantly to screening and data collection; The data accuracy was checked by RT. All discrepancies among them were resolved through consensus or discussion with a third author (MF, RT, or MA). RT contributed significantly to data analysis, data interpretation and manuscript preparation. Author AH, RT, M.A-K and MA and K-B.L contributed significantly to, data interpretation and manuscript preparation. FA contributed to supervising and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was conducted under observation of the Noncommunicable Diseases Research Center of Fasa University of Medical Sciences, Fars, Iran. It was not applicable to obtain consent form.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on COVID-19 and Fungal Infections
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habibzadeh, A., Lankarani, K.B., Farjam, M. et al. Prevalence of Fungal Drug Resistance in COVID-19 Infection: a Global Meta-analysis. Curr Fungal Infect Rep 16, 154–164 (2022). https://doi.org/10.1007/s12281-022-00439-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-022-00439-9