Abstract
Purpose of Review
More than half a billion people have been infected and 6.2 million killed by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) since the start of the pandemic in 2019. Systemic glucocorticoids are a double-edged sword, on the one hand, life-saving in treating COVID-19 complications while on the other hand, potentially leading to life-and-limb-threatening opportunistic fungal infections. Mucormycosis (MM) is caused by the mucormycetes family. Although rare, it is characterized by high mortality and significant morbidity. The gross similarities observed with other fungal infections which respond to different treatment regimens have made it all the more imperative to quickly and sensitively diagnose and treat MM. This review discusses the epidemiology of MM before and during the COVID-19 pandemic, associated risk factors, COVID-19-associated MM, diagnosis, and current therapeutic interventions.
Recent Findings
There has been a widespread and worrisome trend of rising in cases of MM, worldwide, but more so in the Indian subcontinent, where it is nicknamed the “black fungus.” This upsurge has picked up the pace ever since the start of the COVID-19 pandemic. Necrosis is secondary to the angio-invasive and pro-thrombotic nature of the mold resulting in extensive lesions presenting mostly as rhino-orbital MM (ROM) and rhino-orbito-cerebral MM (ROCM). Infection is mostly observed in subjects with underlying risk factors such as uncontrolled diabetes, those receiving hematopoietic stem cell transplant, and/or on corticosteroid or immunosuppressive therapy, although it is widely suspected that other factors such as iron and zinc may play a role in the pathogenesis of MM. The “One world one guideline” strategy advocates both prophylactic anti-fungal therapy along with aggressive, prompt, and individualized treatment with anti-fungal drugs such as amphotericin B in addition to vigorous surgical intervention. High-risk groups need particularly rapid diagnosis although empirical anti-fungal therapy may not be delayed. Speeding diagnostic turnaround times are essential to institute early therapy, and there is much scope for newer modalities such as PCR, matrix-assisted laser desorption ionization-time of flight mass spectrometry, and whole-genome sequencing in such endeavors. The results of strict monitoring of blood glucose levels along with rational and limited use of steroids and immunomodulatory drugs have proven to be a significant preventive measure.
Summary
The significant rise in cases of MM worldwide has necessitated viewing each case with a strong index of suspicion. Adoption of rapid diagnostics, early antifungal therapy, and prompt surgical interventions are essential, while high-risk groups need particular focused care which may include prophylactic anti-fungal therapy, limited steroid use, and meticulous control of the underlying disease. Developing quicker and more sensitive diagnostic modalities has great potential to improve the detection and management of MM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As of April 2022, more than half a billion people had been infected and 6.2 million killed by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). Although multiple therapeutic strategies have been investigated, only few have proven effective in treating COVID-19 patients. MM is a serious albeit uncommon fungal infection caused by a family of mucormycetes (mould) also called as zygomycosis [1]. Mucormycetes are a group of fungi, especially in soil which are associated with decay of organic substances, such as leaves, compost piles, and animal dung. In the soil, they are more widespread in summer and autumn than in spring and winter [2]. There are several other different kinds of fungi that can cause MM; among them, the most common are Rhizopus species and Mucor species. Some of the other examples include Rhizomucor, Syncephalastrum, Cunninghamella, Apophysomyces, Lichtheimia (formerly Absidia), and Saksenaea species [1, 3–5]. The differentiating hallmark of invasive MM is a picture of tissue necrosis due to angioinvasion and subsequent thrombosis. MM of the rhinocerebral system may originate as a sinus infection that can spread into the brain [6]. Such MM is most common in unregulated diabetes and those who have undergone transplantation. The most frequent form of MM in cancer patients and in those who have undergone an organ transplant or stem cell transplant is pulmonary (lung) MM. Gastrointestinal mucormycosis is more frequent in smaller children than in adults, particularly in those born preterm. Dissemination of MM to other parts of the body may occur via the bloodstream. There is predisposition for the brain, but can also affect other organs as well including spleen, heart, and skin [7–10]. Fungi are ubiquitously present, and although, many individuals do not get infected by these fungi. Nonetheless, inhalation of mucormycete spores can lead to lung or sinus infection which can be problematic for diabetics and people with compromised immune systems. Sinus, brain, and lung involvement can be fatal in these vulnerable patient groups. Early indications of infection include a stuffy and bleeding nose, vision disturbances, and evidences of inflammation involving the eyes and eyelids. This may ultimately progress to loss of vision. [1, 7]. Early cases were reported from people with diabetes of longstanding duration admitted in the ICU for management of COVID-19 complications. It was then recommended that diabetes must be treated, steroids avoided, and systematic surgical debridements performed to excise all necrotic tissues. The overwhelming majority of cases were seen in India, which was already facing the major brunt of the second COVID-19 wave, the reports of recalcitrant fungal infection being notoriously nicknamed the “black fungus.” Although considerable attention has been paid to COVID-19 associated pulmonary aspergillosis (CAPA) which is the most widely recognized secondary infection, MM remains relatively less known. [11–13]. Pulmonary MM has increased fatality over time. Control of hyperglycemia, initial liposomal amphotericin B therapy, and surgical intervention are critical to effective MM management. The lack of typical risk factors, such as diabetes mellitus, or a history of organ transplantation, etc., in some cases with COVID-19 associated MM (CAM) has been alarming. Glucocorticoids are widely available, inexpensive, and are shown to decrease death rate in COVID-19 patients with hypoxemia [14]. Unfortunately, this might possibly increase the risk for MM, which shows a need to use them wisely. [15]. It is also suspected that Remdesivir could be causing imbalance in sugar levels, which may then lead to this fungal infestation.
Interestingly, recent onset diabetes in COVID-19 patients has also been observed irrespective of corticosteroid administration, often presenting as rhino-orbital mucormycosis (ROM) (Nair & Adulkar et al. 2021). Particularly, during the COVID-19 pandemic, self-medication with zinc containing supplements may have played some role in the increased incidences of fungal infections, especially given the fact that Zn- depletion in vivo by chelating techniques has been recently considered for ameliorating MM (Leonardelli & Macedo et al.).
Along with glucocorticoids, virus initiated immune dysregulation and the use of tocilizumab, an immunomodulatory drug, could further potentiate the risk of infections in patients. Paranasal MM is commonly on the rise among COVID-19-infected individuals which is likely due to systemic immune alterations as a result of the viral infection [16]. COVID-19-related changes in innate immunity may be due to a decrease in the number of T lymphocytes, CD4-T, and CD8-T cells. Amphotericin B is the drug of choice for the treatment of MM, although nephrotoxicity may limit higher doses. Extensive disease may require consideration of second line therapies. Echinocandins when combined with amphotericin B results in the addition of a polyene backbone that increases the success rate of therapy. Other accepted second-line antifungals include the use of posaconazole, triazoles, and isavuconazole [17]. Often, MM requires surgery to excise infected necrotic tissue.
Epidemiology
Pre-SARS-CoV-2 Pandemic Scenario with Underlying Risk Factors
Establishing a clinical diagnosis of MM is a major challenge that adds to the difficulty of getting a clearer view of disease epidemiology [18]. There are numerous factors narrowing our ability to precisely ascertain the true occurrence of MM [19]. Patients of MM may be missed due to lack of microbiological and histological proof. The incorporation of safer and less invasive molecular techniques for pathogen identification has greatly assisted in the resolution of uncertain clinical scenarios. However, MM was an uncommon disease before the onset of the COVID-19 pandemic, representing 8.3–13% of all fungal infections even in highly vulnerable patients. Post-mortem evaluations suggested that the frequency of MM was 10–50-fold less than aspergillosis or candidiasis. Population-wide studies considering specific high-risk populations indicated that MM incidence was higher in patients with leukemia and recipients of stem cell transplants that are persistently exposed to active agents of Aspergillus [20]. Especially high incidence of invasive pulmonary aspergillosis caused by A. flavus was observed in patients of hematopoietic stem cell transplants and blood cancers in an Iranian study [1]. A major shift in the epidemiology of MM had occurred over the years preceding the pandemic. Another Iranian study reported increase in incidence of MM, mostly of R. arrhizus, from 9.7% in 2008 to 23.7% in 2014, the majority of infections having underlying diabetes and showing sinus involvement [2] Other studies also reported the global rise in infection rates with India and China gradually taking the lead. In India, the frequency of MM is 80 times as high, with pre-pandemic estimates of 0.14 cases per 1000 population. Over the years, a rising trend in the number of patients suffering from this disease from multiple centers in India has been observed. [21–26]. As cases of diabetes surge in the developing nations, so does the vulnerability to MM. [10, 27, 28]. Chakarbati et al. observing MM cases in India over a 10-year period (1990–1999) noticed an increased prevalence rate (19.4% by 1999) and reported an incidence of MM as 12.9 cases/year [22]. Studies reported found an average of 35.6–50 cases per year.[4]. However, other areas apart from India and China have also witnessed a steady rise in cases. A major systematic review studied 600 articles observing a total of 851 cases of MM. In the database, they found Europe leading the list with 290 of the 851 cases (34%), Asia (31%), and America (28%). The remaining cases were from Africa (3%), Australia, and New Zealand (3%) [29]. This major skew in data has been attributed to underreporting from the Asian subcontinent. [27]. Multiple studies from Europe including France, Spain, Belgium, and Switzerland in the recent past have also shown an increasing trend in MM cases. In Spain, an incidence of 0.43 cases/100,000 hospital admissions has been seen. [29–31]. A study from Belgium reported a manifold increase in annual incidence from 0.019 cases per 10,000 patient-days to 0.148 cases per 10,000 patient-days over a period of 10 years [32].
An American population–based study on invasive mycotic infections reported cumulative incidence of 178.3 per million per year [33]. In an American hospital–based study, the prevalence of hospitalizations due to MM was estimated at 0.12 per 10,000 and a rise was noted to about 0.16 per 10,000 discharges from the hospital [34]. Transplant Associated Infection Surveillance Network (TRANSNET) Database on hematopoietic stem cell transplant reported 983 invasive fungal Infections among 875 recipients. [35]. The multicenter Prospective Antifungal Therapy registry (PATH) on invasive fungal infections in hematopoietic stem cell transplant recipients found 250 fungal infections among 234 adult recipients [36]. MM often has a grim mortality rate despite intensive treatment protocols. An overall death rate of 54% was identified in the published cases of MM. Depending on the underlying medical condition, species genera, and body state, the fatality rate differed, the mortality rate being 46% among people with sinus infections, 76% for patients with pulmonary infections, and 96% for disseminated MM. [5]. Specific unique groups of individuals are also highly susceptible to the disease. These include people with cancer, neutropenia, hemochromatosis/iron overload, skin injury caused due to wounds, burns or surgery, low birthweight, and prematurity (neonatal gastrointestinal MM). Apart from the above-mentioned risk factors related to the host, numerous cases have been partially linked to the environment persisting in the hospitals. Nosocomial MM has been linked with an exposure to heavy fungal loads in the air because of contaminated air filters, construction work, and a variety of procedures (healthcare-associated) and devices like transdermal nitrate patches, contaminated dressings, tongue depressors, intravenous catheters, and even allopurinol pills [20]. Deferoxamine (DFO) test, a noninvasive procedure that is used as an iron chelator to treat aluminium/iron overload in patients undergone dialysis, is a reported risk factor for angioinvasive MM. As indicated in a report by the international registry of mycoses, 78% of dialysis patients with MM had undergone DFO therapy. Apart from DFO, overload of iron, either due to dyserythropoiesis or secondary to transfusional, is likely to raise the risk of MM [37].
Other Risk Factors During the Pre-COVID-19 Era
Transplantation
Patients with haematopoietic melanoma and those with AML (acute myelogenous leukemia) are high-risk individuals for MM, with varying incidences which range from 1 to 8% [38]. The disease has a low occurrence rate in other chronic or acute HMs. In recipients of solid organ transplant, MM forms a small proportion of fungal infections. The mortality rate associated with the disease in such cases is higher. Depending on the type of transplant, the incidence ranges from 0.4 to 16%. Liver transplant recipients are found to be at a higher risk of early development of MM after transplantation than renal and heart transplant recipients. A chronic immunosuppression was observed in all the patients, usually when high doses of systemic corticosteroids were given. Furthermore, the spread of fungal infection to distant organs was also observed often after rejection and the treatment of disease [39]. The spread was preferential to soft tissues and skin. In a study, an increased risk was observed in the recipients with diabetes mellitus, renal failure, and prior usage of caspofungin/voriconazole. On the contrary, use of tacrolimus was related with a decreased risk [7].
Diabetes
A predisposing factor to MM is diabetes mellitus in 36–88% cases. Uncontrolled hyperglycemia with the presence of ketoacidosis imparts high risk for infection spread. [40]. In patients with undetected diabetes mellitus, MM could be the prime manifestation. MM is a rare occurrence in those having metabolically controlled diabetes. A study reported the existence of diabetes in 36% out of 929 reported cases of MM. [40, 41]. Rhizopus was the most frequently isolated in this subgroup. The high blood sugar levels seen in uncontrolled diabetes impair function of neutrophils. Ketoacidosis along with hyperglycemia and an acidic environment might lead to a defect in the motility and pathogenic activity of neutrophils. It is probable that with fall in pH, dissociation of iron-protein complexes occurs, which allows the fungal cells to opportunistically use the available increased free iron (Fig. 1). [42, 43].
COVID-19, diabetes, and corticosteroid interactions with mucormycosis. Where COVID-19 is likely to cause hypoxia, lymphopenia increases in the endothelial receptor glucose regulated protein 78 (GRP-78) and spore coat protein homologs of endothelial receptor (Cot-H). In COVID-19-infected individuals with ketoacidosis, excessive corticosteroids, and pre-diabetes and hyperglycemia likely to rise. COVID-19 results in cytokine storm creation (interleukin 6), an increase of free intracellular iron, reactive oxygen species, and opportunistic fungi such as Mucormycosis infiltrate and overpower the defence owing to compromised immune systems
Corticosteroid Use
Chronic use of corticosteroids is yet another major risk factor that increases the susceptibility of a patient to MM [44]. An overuse of corticosteroids causes defects in neutrophils and macrophages in addition to steroid-induced diabetes (Fig. 1). [2]
Current SARS-CoV-2 Pandemic Scenario and Underlying Risk Factors
The successive waves of the COVID-19 pandemic saw the Indian subcontinent receiving the greatest share of MM cases in the world. A multicenter study featuring 16 major hospitals of India in the closing months of 2020 observed that 65.2% of the 287 MM cases were (COVID-19)–associated mucormycosis (CAM). CAM affected significantly more males than females (80.2% vs 19.8%). The prevalence of CAM among hospitalized COVID-19 patients was 0.27%. Interestingly, the number of MM cases had risen more than twofold compared to the previous year. Diabetes was the most frequently found comorbidity in both CAM as well as non-COVID-19 associated MM, the increased glucocorticoid usage probably accounting for the increased numbers. A case fatality rate of 45.7% at 12 weeks for both CAM and non-CAM was observed as well. Irrespective of COVID-19 co-infection, rhino-orbital MM (ROM), and rhino-orbito-cerebral mucormycosis (ROCM) presentations were seen in the overwhelming majority of cases (Patel & Agarwal et al. 2021). A recent review of 80 cases from 18 countries, the majority being obviously from India, revealed a similarly high mortality of 49% attributed to cerebral, pulmonary or disseminated MM. Vision loss affected 46% of survivors. They also had similar findings of underlying uncontrolled diabetes, corticosteroid use, ROCM preponderance (74%), and greater share of males (78%) as reported in the previous study. Thus, the situation in India was characterized by higher ROCM preponderance (98%) compared to other countries, while diabetes was also a more influential risk factor for MM, both these two features coexisting in almost all Indian case studies [45•]. Media reports hinted at 47,000 detected cases present in India, the undetected cases being probably higher. Another study investigating 275 cases of CAM, the majority (84.73%) being again from India, reported a lesser fatality rate in India (36.5%) compared to globally reported cases (61.9%), which was attributed to the comparatively higher incidence of ROM, which is relatively “innocuous” as compared to ROCM. Both ROM and ROCM formed the majority of presentations (64%) reported worldwide, and substantially more (89%) in India. Interestingly, the distribution of ROCM cases in India as reported by this study was at variance with the study by Hoenigl et al., as only 29.6% of cases were of the more severe ROCM form compared to the 98% reported by the latter study. There were lesser reported cases of pulmonary and disseminated MM in India, both being factors associated with increased mortality. Amongst the comorbidities, diabetes was extensively reported worldwide (54.8%), the incidence being higher in India (66.1%). Countries other than India additionally reported underlying history of organ transplantation and hematological malignancies in a substantial number of cases (Muthu & Rudraswamy et al.). A larger study by the Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC) included 2826 patients suffering from ROCM over a 17-month period and observed male preponderance (71%), underlying diabetes (78%) and hypertension (80%) prevalence, steroid administration (87%), but a lower mortality of 14% at follow-up (Sen & Honavar et al.). MM cases had increased several-fold during the second wave of the pandemic in India were seen in a study including 178 cases of post COVID-19 treatment MM reported from a single tertiary care center in southern India during the first 6 months of 2021. Interestingly, the same institution had reported only 7 cases of MM annually for the previous 3 years. Most of the cases were unifocal (39.9%), while ROM and ROCM accounted for 24.2% and 2.2% of cases, respectively. This probably accounted for the lower-case fatality rate (15%) compared to the abovementioned studies. While 73% of the patients had moderate or severe COVID-19, 74.2% had underlying diabetes and 52.8% had received corticosteroids (Joshi & Telang et al., 2022). A study investigating incidence of MM amongst 2567 COVID-19 patients admitted to 3 tertiary care centres in India reported a prevalence of 1.8%. All the 47 patients of MM had previously received corticosteroids, 76.6% had underlying diabetes, and 42.6% required invasive ventilation. The fatality rate was 23.4%, most succumbing within a week of diagnosis (Selarka & Sharma et al., 2021).
A systematic review reported significant preponderance of diabetes mellitus (mostly uncontrolled or poorly controlled) in MM cases followed by hypertension, with lesser frequencies of chronic kidney disease, immunosuppressive therapy, and ischemic heart disease. Most of the cases were associated with corticosteroid use, and more than half of ROCM cases were associated with varying degrees of vision loss (Bhattacharya & Sarma et al. 2021). Another systematic review on ROCM by the same authors mentioned male predominance, widespread underlying diabetes, with poorer glycemic control associated with greater ROCM severity, compounded by corticosteroid use (85.75%). Hypertension and chronic kidney disease were also major underlying comorbidities. Rhizopus was the most widely detected fungal species. Additionally, mechanical ventilation, supplemental oxygen, and broad-spectrum antibiotics were found to be significant risk iatrogenic risk factors in some of the included studies. A mortality rate of 34.4% was however observed (Bhattacharya & Sarma et al. 2021). Interestingly, a study of 5428 COVID-19 patients admitted to a premier hospital in Mumbai, India, over a 14-month period, revealed no cases of MM, either during hospital stay or on follow-up. This, despite 1027 of the cases being admitted to ICUs, 417 having underlying diabetes and 915 having been administered corticosteroids. The authors attributed it to the state government mandated low-dose steroid regimen, meticulous blood glucose control, and minimal usage of immunomodulatory drugs [46••].
Pathogenesis and the Role of Iron and Zinc
Often, the spores of Rhizopus spp are inhaled. Inhalation is in fact the primary route of entry in immunocompromised patients. Infection may also be acquired through open wounds. The spore coat protein cotH, which is ubiquitously present in all Mucorales, initiates the infection cascade by attaching to the host endothelial chaperone, glucose-regulated protein 78(GRP-78), which has a binding preference for mucorales germlings. Spores mature into coenocytic hyphae with initial proliferation in the sinuses, followed by encroachments into the orbit and brain. The invading fungal structures are endocytosed, and it is thought that activation of the platelet-derived growth factor (PDGF) pathway follows. The latter pathway is also activated during epithelial infestations which can involve spores. Predictably, different inhibition experiments on GRP-78 expression and PDGF receptor phosphorylation resulted in observable reductions in damage caused by fungal invasion. A robust inflammatory response ensues eventually killing the host cell. Diabetes and its complications result in an augmented expression of CotH and GRP78, often in proportion to the degree of hyperglycemia. This, in addition to compromised chemotactic responses, results in more aggressive MM. In addition, interesting interactions of the fungi with iron have been seen. Transcriptome analyses have shown the high priority accorded to genes related to iron metabolism. The FTR1 gene coding for the high-affinity iron permease (FTR1) is involved in iron uptake (from extracellular heme) and transport in Mucorales. Knock-down studies of FTR in mice have observed reduced virulence of the fungi. In addition, mechanisms for concentration of iron such as receptors for the uptake of iron, siderophores, iron chelators, and enzymes such as permeases and ferrioxidases have also been observed. Enzymatic action causes reduction of ferric iron to a more absorbable ferrous forms.. The angioinvasive predilection of the fungus maybe explained by the evidence on genomic scrutiny of R. oryzae, revealing homologs of heme oxygenase, which may aid in acquisition of iron from host hemoglobin. Iron depletion has been seen to cause apoptosis in R. oryzae. During the ongoing COVID-19 pandemic, elevated ferritin levels have been used to gauge inflammation and cytokine storms. The excess ferritin has been known to damage hepatic cells and incite apoptotic processes releasing free iron and ferritin extracellularly. Ferritin may also “shed” iron during the process. The pro-inflammatory state disturbs the iron-regulatory protein hepcidin levels. The virus has been shown to attack the 1-β chain of hemoglobin causing dissociation and release of iron into the circulation. Thus, this plentiful supply of the metal is harnessed by Rhizopus spp with all its iron-focused cell machinery to boost growth and spread (Hassan & Voigt et al. 2019, Soare & Watkins et al. 2020, Tabassum & Araf et al. 2021). Zinc also has a suspicious role in MM in COVID-19 patients. Initially, it was included in several treatment regimens of COVID-19 patients but later on proved ineffective. Interestingly, zinc has been a known growth factor for fungi, including Mucorales. Zinc deprivation in the host is known to be a protective mechanism against the fungi, and chelators have been reported to inhibit fungal growth in vitro as well as in vivo. Excess availability in the human body secondary to supplementation may increase risk of MM. (Nath & Baidya, 2021 et al.). A study analyzing isolates of Rhizopus arrhizus from CAM patients showed increased fungal growth in zinc enriched media although zinc levels in CAM and non-CAM patients were not significantly different. Earlier, in an in vitro study, it had also been seen that zinc improved growth of Rhizopus stolonifera and other nutrients such as copper, manganese, and zinc could work more effectively only in the presence of zinc (Muthu & Kumar et al. 2021).
Diagnosis
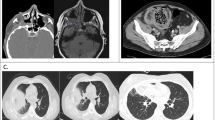
Diagnosing and treatment of MM are challenging. As mentioned above, the disease seems to have increased manifold during the recent COVID-19 pandemic. The explosion in non-communicable and lifestyle-related diseases during the past decade in developing nations is a prime factor in the increased share of MM cases as discussed previously. Longstanding uncontrolled/poorly controlled diabetes and hypertension are the most frequent underlying diseases in third world nations with emerging economies. Suspicious radiological evidences of pulmonary MM include numerous nodules and pleural effusion(s). Computerized tomography (CT) imaging may occasionally reveal the classic “reverse halo sign.” The foundation of diagnostics are microscopy (direct and histopathological) and culture. Molecular techniques can be utilized for the characterization or detecting of mucoromycetes and can be advised to strengthen traditional diagnostic processes as beneficial advancements (Fig. 2).
Clinical Diagnosis
A strong index of suspicion, host identification, and early clinical examination are the requirements for the diagnosis of MM. Invasive mucormycosis is a series of events characterized by fungal angioinvasion and thrombosis resulting in the typical necrotic lesions. Cases of non-COVID-19 pulmonary MM have seen to present with cough and high-grade fever, not responding to broad spectrum antibiotics (Petrikkos, Skiada et al. 2012). Previous studies on non-CAM observed that the presence of diplopia in a diabetic patient should raise strong clinical suspicion of MM (Walsh & Gamaletsou et al., 2012). Sometimes it is possible to clinically differentiate CAM from non-CAM infections. Hypoxemia necessitating ICU admission has been seen with greater frequency in CAM patients compared to non-CAM. Interestingly, teeth and jaw involvement has been observed in CAM, but not in non-CAM admissions. The rest of the clinical presentations, as a whole, are generally similar between CAM and non-CAM. ROM and ROMC presentation preponderance has been reported by several studies, but same is seen with non-CAM cases (Patel & Agarwal et al. 2021) [45•]. Pulmonary forms of mucormycosis are a constant concern in neutropenic patients and patients with graft-versus-host disease. This form of mucormycosis may spread to other nearby organs as well. Diabetic patients generally present with Rhino-orbital or ROC lesions. As far as immunocompetent persons are concerned, cutaneous and soft-tissue MM predominates with little evidence of deeper invasions. Such patients generally present with abscesses and the typical eschars. Diabetic patients seldom develop lung infections. Gastrointestinal involvement is also rare in adults, but is the most common manifestation in neonates (Corniely & Alastruey-Izquierdo, et al. 2019). Studies have reported cases with paralysis of the cranial nerves, diploma, sinus pain, proptosis, edema, orbital apex, and ulcers of the palate. A collection of symptoms and indicators are considered “red flames.” In radiography, pleural MM multiple nodules are detected in greater prevalence [47].
Non-laboratory differentiation from other fungal infections is also possible. In an investigation of 24 patients with lung MM, the CT scan was compared with 96 patients with invasive lung aspergillosis. MM (54%) had higher RHS than aspergillosis (6%, p < 001) but different airway-invasive properties, such as central-lobular nodular cluster stroke, peribronchial, and bronchial wall thickening, were more common in individuals with aspergillosis. Though not definite, these findings can be used as indicators for thorough laboratory diagnostic testing (Jung, Kim et al. 2015). Studies reported a median time from COVID-19 detection to onset of symptoms in the range of 14–28 days. In cases of ROCM, orbital symptoms of pain, (periocular)edema, ptosis, proptosis, ophthalmoplegia, dental symptoms such as loosening and pain, nasal symptoms of pain, eschar, and discharge may also be observed. Particularly, an eschar and surrounding inflammation were utilized in one study for clinical diagnosis of MM. Ocular presentations are mostly unilateral. Varying degrees of vision loss are seen more often than not. Bone penetration was also observed extensively in one study. Generally, vital signs are not adversely affected. (Sen & Honavar et al. 2021, Joshi & Telang et al. 2022, Bhattacharya & Sen et al. 2021) [37, 47]. As already indicated, rhino cerebral, pulmonary, soft tissue, and disseminated illness are the most frequent clinical manifestations of mucoral infection although almost any organ can be affected. Tissue necrosis is an indication of MM; however, diagnosis is sensitive and specific to appearance of lesions. Similar clinical symptoms can occur in other fungi, such as Aspergillus or Fusarium. The positron emission computed tomography (PET/CT) with [18f]-fluorodeoxyglucose seems to be a new imaging technology that may potentially help in diagnosing and managing MM. [48–50]
Microscopy, Histopathology, and Culture
The foundation of MM diagnosis is microscopy (direct and histopathological) and culture of diverse clinical specimens. Direct microscopy of clinical material, preferably utilizing optical illuminators as Blankophor or Calcofluor white, permits a fast diagnosis of MM in clinical specimens [51–53]. Hyphae of Mucorales have an uneven, ribbon-like form, and are non-septate or pauci-septate in width (6 to 25 μm). Hematoxylin and eosin stains may readily show fungal components while Grocott-Gomori methenamine silver colouration can also be used to highlight fungal hyphae and get a clearer picture of morphology. An accurate diagnosis is based on the typical fungal hyphae of mucoromycetes in tissue biopsies or bronchoalveolar lavage (BAL). [23, 54–56]. Tissue histology may reveal inflammation with neutrophils, although the same may not show any findings in those on immunosuppressive medication. In invasive lesions, there may be presence of prominent infarctions and angioinvasions, more so in neutropenic subjects. In cases where nerve structures are damaged, perineural invasion may develop. Histological testing for a valid distinction between the hyphae of Aspergillus and Mucor cannot always be conducted on tissue specifications. Cultures may be preferred, as Mucorales grow rapidly on media such as Sabouraud and PDA agar, at 25 to 30 °C. Nonetheless, tissue identification is an extremely important diagnostic approach since it distinguishes fungi as a pathogen in the collection from a culture contaminant [23, 56].
Drawbacks of Traditional Diagnostic Methods
Culture methods for MM have been notoriously time-consuming with low sensitivity and species specificity. In the case of pulmonary MM, a study compared sensitivity of culture techniques with quantitative polymerase chain reaction (qPCR) assays and reported dismal performance of the former as compared to the latter. Out of the 24 patients of MM detected by PCR of BAL specimens and diagnosis eventually confirmed clinically, only 2 had a positive Mucorales culture. Prompt differentiation of MM from other fungal infections like aspergillosis, which has a totally different line of treatment, would not have been feasible with culture-based methods (Scherer et al. 2018). There are no differentiating biomarkers known. Immunohistochemical methods do not differentiate at the species level. Currently, morphological identification from cultures is available in most centers, especially in developing countries like India. However, the fragility of aseptate hyphae predisposes to damage during histopathological analysis, causing marked reduction in sensitivity. Currently, there is a need to have a laboratory diagnosis which has a short turn-around time and sufficient specificity so that valuable time may be saved in initiating a suitable anti-fungal regimen (Soare & Watkins et al.2020).
New Diagnostic Methods
Different molecular methods have emerged both for bodily fluids and tissue specimens for prompt MM diagnosis (Fig. 2). Among them are the polymerase chain (PCR)-based technologies, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF), PCR-electrospray ionization mass spectrometry (PCR-ESI/MS), high-resolution melt analysis (HRMA), and breath-based-metabolomic and metagenomic shotgun test [57–62]
Other early/fast diagnostic methods of MM include enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay (LFIA) [62–64].
Polymerase Chain Reaction
PCR testing is of importance in early diagnosis of Mucorales in high-risk patients. In order to diagnose Mucorales, many molecular objectives and targets are used, and different body fluids such as serum, whole blood, BAL, and CSF can be analyzed. The target gene selection for PCR is of essence to maintain efficiency of this procedure. The “Internal transcribed Spacer (ITS)” region of fungal DNA has been of particular interest and the sequence of base-pairs in this region has enabled the designing of multiplex PCR’s capable of detecting various strains responsible for MM. 28S and 18S ribosomal RNA has also been targeted for amplification. PCR has also been suggested as a surrogate for assessment of fungal burden. [64–66].
MALDI-TOF
Another promising culture-backed diagnostic approach to identify Mucormycetes is MALDI-TOF MS). The identification is based on an analysis, in the form of a species-specific spectrum recognized in the data base spectrum of the treated and unknown microbial cells as a micro-protein fingerprint. The optimum mass spectrum identification technique for moulds is simple, resistant to culture changes, repeatable, and cost-effective for most microorganisms [64, 67–69].
Whole Gene Sequencing
The nuclear sequencing process (whole genome and next-generation sequence) has revolutionized the field of infectious diseases, but relatively few advancements have been attained in mucorales research [70]. These procedures have the potential to compare host mycobiomes at baseline with that seen after chemotherapy. However, the absence of Mucorales annotated genomes is still a major restriction.[71].
Serial Detection of Circulating Mucorales DNA
Different assays for detection of DNA of Mucorales in peripheral blood have been developed. Millon et al. has used PCR for Mucor/Rhizopus, Lichtheimia, and Rhizomucor. In the approach used by Springer et al., specific fragments of the 18S and 28S genes are targeted. The amplicons of the 18S gene need to be sequenced for further identification of the genus. These assays were able to detect mucormycosis before the diagnosis was made by histopathology or culture. In patients with a positive blood PCR, the molecular test preceded the conventional tests in 80–90% of patients and was positive up to 250 days earlier. This makes these tests attractive as screening assays, as well as a diagnostic test in cases in which more invasive sampling is not feasible.
Treatment of Mucormycosis
The core principles of MM therapy include risk stratification for disease severity, and intensive pre-diagnosis efforts in the clinical and laboratory areas, and timely initiation of an effective antifungal therapy (monotherapy or combined therapy) together with aggressive surgical debriding of necrotic lesions [72]. Early detection and early treatment can avoid gradual invasion of tissue, consequent deformity, lessen the need for significant surgery, and increase survival. A multimodal approach, including redressal of underlying predisposing factors, early administration of active antifungal agents at the optimal dose, complete removal of all affected tissues, and the use of individualized additional treatments, is the foundation of successful management of MM. [72–75]
Antifungals, Triazoles, and Combination Therapy
Only the first-line treatment for MM has been explored for amphotericin B (AMB) and its lipid preparations, and lately isavuconazole [76]. For primary MM therapy, AMB is regarded as the anti-fungal of choice. In both laboratory (in vitro and in vivo) as well as in-clinical trials, the effectiveness of AMB has been demonstrated. The appropriate dose for AMB and its MM formulations has been topic of debate. In keeping with current standards, the recommended daily dosage of liposomal amphotericin B (LAmB) and amphotericin B lipid complex (ABLC) is 5 mg/kg/day. On the other hand, greater nephrotoxicity and electrolyte derangements were related with high dosage LAmB. Typically, a 40% doubling of baseline creatinine in individuals, mandating dosage reduction [77, 78], has been observed. While dosages beyond 5 mg/kg/day have not demonstrated to be more effective for MM, individual doses might be explored, in particular when CNS or osteoarticular involvement occurs [58, 79]. Fluconazole, itraconazole, and voriconazole show little or no anti-Mucorales activity among triazole antifungals. There is evidence that the newer triazoles, namely, posaconazole and Isavuconazole, are more effective in vitro in Mucorales [76, 80].
Notwithstanding, a lack of strong clinical evidence, treatment in severely immunocompromised patients has been widely employed using a combination of antifungals. The advantages of this “multi-drug” approach include several synergistic effects in drug activity [81]. There is inconsistent information on the effectiveness of the AMB + triazole combination in the treatment of MM. The combination of polyene and posaconazole was effective in ex-vivo studies, while in vivo investigations in murine MM models revealed no advantage when administered simultaneously [82–85]. 32 patients in one retrospective case series were treated with combination of polyene with posaconazole, including 32 patients of MM with hematologic or aplastic anemia not responding to previous treatment (primarily with LAmB). 18 patients (56%) saw clinical improvement after 3 months of therapy. [72]
Newer Treatment Guidelines
The “One World One Guideline” proposed by the European Confederation of Medical Mycology (ECMM) have suggested prompt surgical intervention, early in the course of confirmed disease, in addition to pharmacological approaches. Pharmacotherapeutic prophylaxis utilizing Posaconazole with either delayed-release moderate strength dose or oral suspension with marginal strength dose has been recommended in immunocompromised patients. In this category of patients, any prior history of MM necessitates a sturdy surgical approach. Anti-fungals to which the patient had been responding in the previous infection may need to be re-employed. In case fever of unknown origin is the sole source of infection, a MM focused regimen is discouraged. Suspected MM in immunocompromised patients should be confirmed as quickly as possible, but institution of anti-fungal treatment should not be delayed in any case. First line monotherapy should be initiated with LAmB at 5–10 mg/kg/day. Evidence of renal toxicity may be tackled by reducing doses below 5 mg/kg/day with marginal strength. Medications are to uniformly administer at full dose from first day of therapy itself. Patients without CNS involvement can be administered moderate strength ABLC at 5 mg/kg/day. Amphotericin B deoxycholate use is not recommended in the presence of available alternatives. Moderate strength Isavuconazole can also be used as a first-line treatment for MM. Posaconazole oral suspension received marginal support in the guidelines, while its delayed release and IV forms received moderate support for inclusion in the list of first-line drugs. Recommendations for combination therapy showed marginal support for various combinations of polyenes with azoles or echinocandins. In the case of salvage treatment, Isavuconazole and delayed release/infusions of Posaconazole were strongly supported. In case these two drugs proved ineffective, varying strengths of lipid-based Amphotericin B could be used. All measures should be continued till immunosuppression ceases. Intravenous treatment maybe instituted till stabilization is achieved, following which oral treatment with delayed release Isavuconazole or Posaconazole is strongly recommended.
Surgical Interventions
The cornerstone of MM treatment is necrotic tissue removal surgery [74]. In people with rhino-orbital MM, magnetic resonance imaging may be used in staging resectability of lesions. Surgical removal of contaminated tissue is also critical in the treatment of rhino-orbital brain disease [86–89••]. However, the influence of the procedure on findings is difficult to discern because of the selection biases.
Conclusion
MM is an aggressive, yet underreported complication with an alarming incidence of mortality and serious morbidities. However, true etiology varies globally, and medical professionals, especially in developing nations have difficulties in identifying this ailment. Nonetheless, much evidence points to a rise in cases since the beginning of the COVID-19 pandemic. The widespread adoption of corticosteroids, for the treatment of viral complications, on a background of uncontrolled diabetes, is widely believed to be contributory to the resurgence in MM cases. Thus, every effort must be made to maintain euglycemia and rationalize corticosteroids administration in such patients. The diagnosis of MM is of essence as different fungal infections may have similar presentations despite different treatment protocols. Prompt treatment with anti-fungal drugs such as amphotericin B and meticulous surgical intervention is vital to safeguard from potentially fatal complications and life-changing morbidities. High-risk groups should receive anti-fungal treatment without delay, while all efforts must be made for quick diagnosis of MM. Developments in modalities such as PCR, MALDI-TOF, and WGS have the potential to drastically improve diagnostic capabilities, while rational steroid, immunomodulatory drug administration, and strict glucose monitoring have demonstrated evidence of minimizing the serious burdens posed by MM.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15:2–9.
Nogueira EL, et al. Mucormycosis may mimic disease relapse in Wegener’s granulomatosis. J Rheumatol. 2010;37(6):1364–5.
Al-Ajam M, et al. Mucormycosis in the Eastern Mediterranean: a seasonal disease. Epidemiol Infect. 2006;134(2):341–6.
Sivagnanam S, et al. Seasonal clustering of sinopulmonary mucormycosis in patients with hematologic malignancies at a large comprehensive cancer center. Antimicrob Resist Infect Control. 2017;6(1):1–11.
Roden MM, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53.
Hanley B, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–53.
Song Y, et al. Mucormycosis in renal transplant recipients: review of 174 reported cases. BMC Infect Dis. 2017;17(1):1–6.
Ahmed A, et al. Environmental occurrence of Madurella mycetomatis, the major agent of human eumycetoma in Sudan. J Clin Microbiol. 2002;40(3):1031–6.
Vallabhaneni S, Mody RK. Gastrointestinal mucormycosis in neonates: A review. Curr Fungal Infect Rep. 2015;9(4):269–74.
Francis JR, et al. Mucormycosis in children: review and recommendations for management. J Pediatr Infect Dis Soc. 2018;7(2):159–64.
Arastehfar A, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi. 2020;6(2):91.
Koehler P, Bassetti M, Chakrabarti A, Chen SC, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. The Lancet Infectious Diseases. 2021;21(6):e149–62.
Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP. Navigating the uncertainties of COVID-19–associated Aspergillosis: a comparison with influenza-associated Aspergillosis. J Infect Dis. 2021;224(10):1631–40.
Sterne JA, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41.
Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–92.
Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5-264.e8.
Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12(3):85.
De Pauw B, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46(12):1813–21.
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442–7.
Petrikkos G, et al. Mucormycosis: ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis. 2003;22(12):753–6.
Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):523.
Chakrabarti A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect. 2001;42(4):261–6.
Chakrabarti A, et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Sabouraudia. 2006;44(4):335–42.
Chakrabarti A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85(1009):573–81.
Chakrabarti A, Dhaliwal M. Epidemiology of mucormycosis in India. Curr Fungal Infect Rep. 2013;7(4):287–92.
Prakash H, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):395–402.
Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1):26.
Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265.
Jeong W, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34.
Guinea J, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PLoS ONE. 2017;12(6):e0179136.
Torres-Narbona M, et al. Impact of zygomycosis on microbiology workload: a survey study in Spain. J Clin Microbiol. 2007;45(6):2051.
Saegeman V, et al. Increasing incidence of mucormycosis in University Hospital, Belgium. Emerg Infect Dis. 2010;16(9):1456.
Rees JR, et al. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27(5):1138–47.
Kontoyiannis DP, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. 2016;16(1):1–6.
Kontoyiannis DP, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–100.
Neofytos D, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–73.
Petrikkos G, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S23–34.
Funada H, Matsuda T. Pulmonary mucormycosis in a hematology ward. Intern Med. 1996;35(7):540–4.
Fishman J. Infection in organ transplantation. Am J Transplant. 2017;17(4):856–79.
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4). 102146
Lanternier F, Lortholary O. Zygomycosis and diabetes mellitus. Clin Microbiol Infect. 2009;15:21–5.
Rammaert B, et al. Diabetes and mucormycosis: a complex interplay. Diabetes Metab. 2012;38(3):193–204.
Asghar SA, Majid Z, Tahir F, Qadar LT, Mir S. Rhino-oculo cerebral mucormycosis resistant to amphotericin B in a Young patient with diabetic ketoacidosis. Cureus. 2019;11(3).
Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood J Am Soc Hematol. 2011;118(5):1216–24.
• Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022. https://doi.org/10.1016/S2666-5247(21)00237-8. This study emphasizes that mucormycosis is associated with high morbidity (often of the life-changing category), and high mortality. Underlying complaints of poor glycemic control and systemic corticosteroid use are a common feature in such patients, while the most common clinical presentation appears to be of rhino-orbital involvement. Problems with diagnosis especially in developing countries such as India make it more challenging to timely treat the fungal ailment.
•• Mulakavalupil B, Vaity C, Joshi S, Misra A, Pandit RA. Absence of Case of Mucormycosis (March 2020-May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit. Diabetes Metab Syndr: Clin Res Rev [Internet]. 2021;15(4):102169. https://doi.org/10.1016/j.dsx.2021.06.006. This study of more than 1000 COVID-19 ICU admissions reported no MM cases which could be possibly attributed to the local state government regulations enforcing low-dose steroid regimen, avoidance of immune-modifying drugs, and meticulous blood glucose monitoring.
Rodrıguez-Zulueta P, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. n.d.
Legouge C, et al. The reversed halo sign: pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin Infect Dis. 2014;58(5):672–8.
Jung J, et al. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect. 2015;21(7):684.e11-684.e18.
Liu Y, et al. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38(9):e370–1.
Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125(3):375–8.
Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15:60–5.
Lass-Flörl C, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis. 2007;45(7):e101–4.
Lackner M, Caramalho R, Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol. 2014;9(5):683–95.
Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236.
Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247.
McCarthy MW, Walsh TJ. PCR methodology and applications for the detection of human fungal pathogens. Expert Rev Mol Diagn. 2016;16(9):1025–36.
Cornely O, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20:5–26.
Zaman K, et al. Molecular diagnosis of rhino-orbito-cerebral mucormycosis from fresh tissue samples. J Med Microbiol. 2017;66(8):1124–9.
Alanio A, et al. Molecular identification of Mucorales in human tissues: contribution of PCR electrospray-ionization mass spectrometry. Clin Microbiol Infect. 2015;21(6):594.e1-594.e5.
Lengerova M, Racil Z, Hrncirova K, Kocmanova I, Volfova P, Ricna D, et al. Rapid detection and identification of mucormycetes in bronchoalveolar lavage samples from immunocompromised patients with pulmonary infiltrates by use of high-resolution melt analysis. J Clin Microbiol. 2014;52(8):2824–8.
Cassagne C, et al. Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS ONE. 2011;6(12):e28425.
Orne C et al. Cell wall fucomannan is a biomarker for diagnosis of invasive murine mucormycosis. Proceedings of the 28th ECCMID, Madrid, Spain. 2018;21–24.
Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18(10):845–54.
Dannaoui E. Molecular tools for identification of Zygomycetes and the diagnosis of zygomycosis. Clin Microbiol Infect. 2009;15:66–70.
Walther G, et al. DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia: Mol Phylogeny Evol Fungi. 2013;30:11.
Marvin LF, Roberts MA, Fay LB. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in clinical chemistry. Clin Chim Acta. 2003;337(1–2):11–21.
Becker PT, et al. Identification of filamentous fungi isolates by MALDI-TOF mass spectrometry: clinical evaluation of an extended reference spectra library. Med Mycol. 2014;52(8):826–34.
Giebel R, et al. Microbial fingerprinting using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS): applications and challenges. Adv Appl Microbiol. 2010;71:149–84.
Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21(7):334–41.
Liu M, et al. Comparative genome-wide analysis of extracellular small RNAs from the mucormycosis pathogen Rhizopus delemar. Sci Rep. 2018;8(1):1–10.
Skiada A, et al. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):S93–101.
Peixoto D, et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol. 2014;52(3):1016.
Ervens J, et al. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. 2014;42(2):429–32.
Graves B, et al. Isavuconazole as salvage therapy for mucormycosis. Med Mycol Case Rep. 2016;11:36–9.
Marty FM, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–37.
Gamaletsou MN, et al. Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep. 2012;14(4):423–34.
Walsh TJ, et al. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26(6):1383–96.
Lanternier F, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015;70(11):3116–23.
Nagappan V, Deresinski S. Posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45(12):1610–7.
Spellberg B, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis. 2012;54(suppl_1):S73–8.
Ballester F, Pastor FJ, Guarro J. In vitro activities of combinations of amphotericin B, posaconazole and four other agents against Rhizopus. J Antimicrob Chemother. 2008;61(3):755–7.
Bernardo RM, et al. Therapeutic challenges of hepatic mucormycosis in hematologic malignancy: a case report and review of the literature. Am J Case Rep. 2016;17:484.
Ville S, et al. Disseminated mucormycosis with cerebral involvement owing to rhizopus microsporus in a kidney recipient treated with combined liposomal amphotericin B and posaconazole therapy. Exp Clin Transplant: Off J Middle East Soc Organ Transplant. 2014;14(1):96–9.
Lebeau O, et al. Disseminated Rhizopus microsporus infection cured by salvage allogeneic hematopoietic stem cell transplantation, antifungal combination therapy, and surgical resection. Transpl Infect Dis. 2010;12(3):269–72.
Zuniga MG, Turner JH. Treatment outcomes in acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):242–8.
Gillespie MB, O’Malley BW, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol-Head Neck Surg. 1998;124(5):520–6.
Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clin. 2016;30(1):143–63.
•• Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen S, Dannaoui E, Hochhegger B et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium A. Lancet Infect Dis [Internet]. 2019;19(12):1–34. Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(19)30312-3/fulltext. https://doi.org/10.1016/S1473-3099(19)30312-3. Global The “One World One Guideline,” proposed by the European Confederation of Medical Mycology (ECMM) have suggested prompt surgical intervention, early in the course of confirmed disease. Recognizing disease patterns is essential. It is strongly recommended to begin initial treatment with high-dose liposomal amphotericin B, while I.V. isavuconazole and posaconazole may also be tried.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on COVID-19 and Fungal Infections
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mansoor, S., Ahmed, T.I., Happa, K. et al. Spectrum of Mucormycosis Before and During COVID-19: Epidemiology, Diagnosis, and Current Therapeutic Interventions. Curr Fungal Infect Rep 16, 131–142 (2022). https://doi.org/10.1007/s12281-022-00438-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-022-00438-w