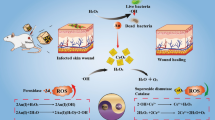
Abstract
Infectious microbes that spread easily in healthcare facilities remain as the severe threat for the public health, especially among immunocompromised populations. Given the intricate problem of dramatic increase in resistance to common biocides, the development of safe and efficient biocide formulated agents to alleviate drug resistance is highly demanding. In this study, Schiff-base ligands were successfully formed on natural biopolymer of epsilon-poly-L-lysine (ε-PL) decorated aldehyde functionalized mesoporous silica SBA-15 (CHO-SBA-15) for the selective coordination of silver ions, which was affirmed by various physicochemical methods. Besides the identified broad-spectrum antibacterial activities, the as-prepared Schiff-base silver nanocomplex (CHO-SBA-15/ε-PL/Ag, CLA-1) exhibited an improved inhibitory effect on infectious pathogen growth typified by Escherichia coli and Staphylococcus aureus in comparison with two control silver complexes without Schiff-base conjugates, SBA-15/ε-PL/Ag and CHO-SBA-15/Ag, respectively. In addition, CLA-1 remarkably inhibited the growth of Mycobacterium tuberculosis due to the excellent antimicrobial activity of silver species. Significantly, CLA-1 kills Candida albicans cells, inhibits biofilm formation, and eliminates preformed biofilms, with no development of resistance during continuous serial passaging. The antifungal activity is connected to disruption of bacterial cell membranes and increased levels of intracellular reactive oxygen species. In mouse models of multidrug-resistant C. albicans infection, CLA-1 exhibited efficient in vivo fungicidal efficacy superior to two antifungal drugs, amphotericin B and fluconazole. Moreover, CLA-1 treatment induces negligible toxicity against normal tissues with safety. Therefore, this study reveals the pivotal role of the molecular design of Schiff-base silver nanocomplex formation on biopolymer surface-functionalized silica mesopores as a green and efficient nanoplatform to tackle infectious microbes.

Similar content being viewed by others
References
WHO. Global tuberculosis report 2018. WHO: Geneva, 2018.
Mukherjee, K.; Tribedi, P.; Mukhopadhyay, B.; Sil, A. K. Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol. Lett. 2013, 338, 177–183.
Gonçalves, B.; Ferreira, C.; Alves, C. T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Grit. Rev. Microbiol. 2016, 42, 905–927.
Brown, G D.; Denning, D. W.; Gow, N. A. R.; Levitz, S. M.; Netea, M. G.; White, T. C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13.
Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J. L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6.
Lohse, M. B.; Gulati, M.; Johnson, A. D.; Nobile, C. J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31.
Zarnowski, R.; Sanchez, H.; Covelli, A. S.; Dominguez, E.; Jaromin, A.; Bernhardt, J.; Mitchell, K. F.; Heiss, C.; Azadi, P.; Mitchell, A. et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoSBiol. 2018, 16, e2006872.
Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961.
Król, A.; Pomastowski, P.; Rafinska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52.
Arias, L. S.; Pessan, J. P.; Miranda Vieira, A. P.; Toito de Lima, T. M.; Botazzo Delbem, A. C.; Monteiro, D. R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46.
Arzhakova, O. V.; Dolgova, A. A.; Volynskii, A. L. Mesoporous and nanocomposite fibrous materials based on poly(ethylene terephthalate) fibers with high craze density via environmental crazing: Preparation, structure, and applied properties. ACS Appl. Mater. Interfaces 2019, 11, 18701–18710.
Wu, J. H.; Li, F. Y.; Hu, X.; Lu, J. X.; Sun, X. L.; Gao, J. Q.; Ling, D. S. Responsive assembly of silver nanoclusters with a biofilm locally amplified bactericidal effect to enhance treatments against multi-drug-resistant bacterial infections. ACS Cent. Sci. 2019, 5, 1366–1376.
Siddiqi, K. S.; Husen, A.; Rao, R. A. K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14.
BurduŞel, A. C.; Gherasim, O.; Grumezescu, A. M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681.
Song, Y. Y.; Jiang, H. J.; Wang, B. B.; Kong, Y.; Chen, J. Silver-incorporated mussel-inspired polydopamine coatings on mesoporous silica as an efficient nanocatalyst and antimicrobial agent. ACS Appl. Mater. Interfaces 2018, 10, 1792–1801.
Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L. M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2010, 22, 1182–1195.
Hoang, T. T.; Cao, V. D.; Nguyen, N. Q.; Hoang, D. T.; Ngo, V. C.; Nguyen, D. H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 2019, 99, 631–656.
Lu, M. M.; Ge, Y. R.; Qiu, J.; Shao, D.; Zhang, Y.; Bai, J.; Zheng, X.; Chang, Z. M.; Wang, Z.; Dong, W. F. et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int. J. Nanomedicine 2018, 13, 7697–7709.
Liu, J.; Li, S. H.; Fang, Y. S.; Zhu, Z. L. Boosting antibacterial activity with mesoporous silica nanoparticles supported silver nanoclusters. J. Colloid Interface Sci. 2019, 555, 470–479.
Liong, M.; France, B.; Bradley, K. A.; Zink, J. I. Antimicrobial activity of silver nanocrystals encapsulated in mesoporous silica nanoparticles. Adv. Mater. 2009, 21, 1684–1689.
Dai, C. L.; Yuan, Y.; Liu, C. S.; Wei, J.; Hong, H.; Li, X. S.; Pan, X. H. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials 2009, 30, 5364–5375.
Marinescu, G.; Culita, D. C.; Romanitan, C.; Somacescu, S.; Ene, C. D.; Marinescu, V.; Negreanu, D. G.; Maxim, C.; Popa, M.; Marutescu, L. et al. Novel hybrid materials based on heteroleptic Ru(III) complexes immobilized on sba-15 mesoporous silica as highly potent antimicrobial and cytotoxic agents. Appl. Surf. Sci. 2020, 520, 146379.
Isaacs, M. A.; Barbero, B.; Durndell, L. J.; Hilton, A. C.; Olivi, L.; Parlett, C. M. A.; Wilson, K.; Lee, A. F. Tunable silver-functionalized porous frameworks for antibacterial applications. Antibiotics 2018, 7, 55.
Sun, J. M.; Ma, D.; Zhang, H.; Liu, X. M.; Han, X. W.; Bao, X. H.; Weinberg, G.; Pfänder, N.; Su, D. S. Toward monodispersed silver nanoparticles with unusual thermal stability. J. Am. Chem. Soc. 2006, 128, 15756–15764.
Min, S. H.; Yang, J. H.; Kim, J. Y.; Kwon, Y. U. Development of white antibacterial pigment based on silver chloride nanoparticles and mesoporous silica and its polymer composite. Micropor. Mesopor. Mater. 2010, 128, 19–25.
Kostova, I.; Saso, L. Advances in research of schiff-base metal complexes as potent antioxidants. Curr. Med. Chem. 2013, 20, 4609–4632.
Saad, A.; Cabet, E.; Lilienbaum, A.; Hamadi, S.; Abderrabba, M.; Chehimi, M. M. Polypyrrole/Ag/mesoporous silica nanocomposite particles: Design by photopolymerization in aqueous medium and antibacterial activity. J. Taiwan Inst. Chem. Eng. 2017, 80, 1022–1030.
Saad, A.; Vard, C.; Abderrabba, M.; Chehimi, M. M. Triazole/triazine-functionalized mesoporous silica as a hybrid material support for palladium nanocatalyst. Langmuir 2017, 33, 7137–7146.
Saad, A.; Snoussi, Y.; Abderrabba, M.; Chehimi, M. M. Ligand-modified mesoporous silica SBA-15/silver hybrids for the catalyzed reduction of methylene blue. RSC Adv. 2016, 6, 57672–57682.
Saad, A.; Bakas, I.; Piquemal, J. Y.; Nowak, S.; Abderrabba, M.; Chehimi, M. M. Mesoporous silica/polyacrylamide composite: Preparation by UV-graft photopolymerization, characterization and use as hg(II) adsorbent. Appl. Surf. Sci. 2016, 367, 181–189.
Joseph, J.; Rani, G. A. Antioxidant and biochemical activities of mixed ligand complexes. Appl. Biochem. Biotechnol. 2014, 172, 867–890.
Parveen, S.; Velmurugan, G.; Sinn, E.; Venuvanalingam, P.; Govindarajan, S. Water-soluble cobalt(II) & cobalt(III) complexes supported by new triazine Schiff base ligands: Synthesis, structure and biological evaluation. J. Photochem. Photobiol. B: Biol. 2018, 189, 152–164.
Cao, Q.; Yang, J.; Zhang, H.; Hao, L.; Yang, G. G.; Ji, L. N.; Mao, Z. W. Traceable in-cell synthesis and cytoplasm-to-nucleus translocation of a zinc Schiff base complex as a simple and economical anticancer strategy. Chem. Commun. 2019, 55, 7852–7855.
Chow, M. J.; Licona, C.; Wong, D. Y. Q.; Pastorin, G.; Gaiddon, C.; Ang, W. H. Discovery and investigation of anticancer ruthenium-arene Schiff-Base complexes via water-promoted combinatorial three-component assembly. J. Med. Chem. 2014, 57, 6043–6059.
Low, M. L.; Maigre, L.; Dorlet, P.; Guillot, R.; Pages, J. M.; Crouse, K. A.; Policar, C.; Delsuc, N. Conjugation of a new series of dithiocarbazate Schiff base copper(II) complexes with vectors selected to enhance antibacterial activity. Bioconjugate Chem. 2014, 25, 2269–2284.
Kareem, A.; Laxmi; Arshad, M.; Nami, S. A. A.; Nishat, N. Herbomineral based Schiff base ligand and its metal complexes: Synthesis, characterization, catalytic potential and biological applications. J. Photochem. Photobiol. B: Biol. 2016, 160, 163–171.
Dai, T. J.; Wang, C. P.; Wang, Y. Q.; Xu, W.; Hu, J. J.; Cheng, Y. Y. A nanocomposite hydrogel with potent and broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173.
Naz, A.; Arun, S.; Narvi, S. S.; Alam, M. S.; Singh, A.; Bhartiya, P.; Dutta, P. K. Cu(II)-carboxymethyl chitosan-silane Schiff base complex grafted on nano silica: Structural evolution, antibacterial performance and dye degradation ability. Int. J. Biol. Macromol. 2018, 110, 215–226.
Yoshida, T.; Nagasawa, T. ε-poly-L-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26.
Mondragón, L.; Mas, N.; Ferragud, V.; de la Torre, C.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F.; Amorós, P.; Pérez-Payá, E.; Orzáez, M. Enzyme-responsive intracellular-controlled release using silica mesoporous nanoparticles capped with e-poly-L-lysine. Chem. Eur. J. 2014, 20, 5271–5281.
Richards, S. J.; Isufi, K.; Wilkins, L. E.; Lipecki, J.; Fullam, E.; Gibson, M. I. Multivalent antimicrobial polymer nanoparticles target mycobacteria and Gram-negative bacteria by distinct mechanisms. Biomacromolecules 2018, 19, 256–264.
Izzo, L.; Matrella, S.; Mella, M.; Benvenuto, G.; Vigliotta, G. Escherichia coli as a model for the description of the antimicrobial mechanism of a cationic polymer surface: Cellular target and bacterial contrast response. ACS Appl. Mater. Interfaces 2019, 11, 15332–15343.
Zhou, C. C.; Li, P.; Qi, X. B.; Sharif, A. R. M.; Poon, Y. F.; Cao, Y.; Chang, M. W.; Leong, S. S. J.; Chan-Park, M. B. A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-L-lysine. Biomaterials 2011, 32, 2704–2712.
Wang, R.; Li, Q.; Chi, B.; Wang, X. Q.; Xu, Z.; Xu, Z. Q.; Chen, S.; Xu, H. Enzyme-induced dual-network e-poly-L-lysine-based hydrogels with robust self-healing and antibacterial performance. Chem. Commun. 2017, 53, 4803–4806.
Velikova, N.; Mas, N.; Miguel-Romero, L.; Polo, L.; Stolte, E.; Zaccaria, E.; Cao, R.; Taverne, N.; Ramón Murguía, J.; Martinez-Manez, R. et al. Broadening the antibacterial spectrum of histidine kinase autophosphorylation inhibitors via the use of ε-poly-L-lysine capped mesoporous silica-based nanoparticles. Nanomed.: Nanotechnol. Biol. Med. 2017, 13, 569–581.
Amariei, G.; Kokol, V.; Vivod, V.; Boltes, K.; Letón, P.; Rosal, R. Biocompatible antimicrobial electrospun nanofibers functionalized with e-poly-L-lysine. Int. J. Pharm. 2018, 553, 141–148.
Ghilini, F.; Rodríguez González, R. C.; Miñán, A. G.; Pissinis, D.; Hernández Creus, A.; Salvarezza, R. C.; Schilardi, P. L. Highly stabilized nanoparticles on poly-L-lysine-coated oxidized metals: A versatile platform with enhanced antimicrobial activity. ACS Appl. Mater. Interfaces 2018, 10, 23657–23666.
Song, Y. Y.; Zhu, P.; Wu, Y.; Tan, L.; Wei, W.; Liu, S. Q.; Huang, Q.; Chen, J. Epsilon-poly-L-lysine decorated ordered mesoporous silica contributes to the synergistic antifungal effect and enhanced solubility of a lipophilic drug. Mater. Sci. Eng.: C 2019, 99, 231–240.
Kruk, M.; Jaroniec, M.; Ko, C. H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968.
Yang, F.; Song, Y. Y.; Cai, L.; Zhou, S. J.; Chen, J.; Kong, Y. Enriched Ag nanospecies interspersed nanoporous siliceous antibacterial agent. ChemistrySelect 2018, 3, 10255–10258.
Song, Y. Y.; Cai, L.; Tian, Z. C.; Wu, Y.; Chen, J. Phytochemical curcumin-coformulated, silver-decorated melanin-like polydopamine/mesoporous silica composites with improved antibacterial and chemotherapeutic effects against drug-resistant cancer cells. ACS Omega 2020, 5, 15083–15094.
Zhao, C. L.; Hou, P.; Ni, J. H.; Han, P.; Chai, Y. M.; Zhang, X. N. Ag-incorporated FHA coating on pure mg: Degradation and in vitro antibacterial properties. ACS Appl. Mater. Interfaces 2016, 8, 5093–5103.
Franzblau, S. G.; Witzig, R. S.; McLaughlin, J. C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M. T.; Cook, M. B.; Quenzer, V. K.; Ferguson, R. M. et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate alamar blue assay. J. Clin. Microbiol. 1998, 36, 362–366.
Xu, J.; Wang, B.; Hu, M. H.; Huo, F. M.; Guo, S. C.; Jing, W.; Nuermberger, E.; Lu, Y. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00239–17.
Gillum, A. M.; Tsay, E. Y. H.; Kirsch, D. R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182.
Lin, S. M.; Sin, W. L. W.; Koh, J. J.; Lim, F.; Wang, L.; Cao, D. R.; Beuerman, R. W.; Ren, L.; Liu, S. P. Semisynthesis and biological evaluation of xanthone amphiphilics as selective, highly potent antifungal agents to combat fungal resistance. J. Med. Chem. 2017, 60, 10135–10150.
Yang, M.; Du, K. Y.; Hou, Y. R.; Xie, S.; Dong, Y.; Li, D. R.; Du, Y. H. Synergistic antifungal effect of amphotericin b-loaded poly(lactic-co-glycolic acid) nanoparticles and ultrasound against Candida albicans biofilms. Antimicrob. Agents Chemother. 2019, 63, e02022–18.
Rodrigues, C. E.; Alves, D. E.; Henriques, M. Combination of posaconazole and amphotericin B in the treatment of Candida glabrata biofilms. Microorganisms 2018, 6, 123.
Ramage, G.; vande Walle, K.; Wickes, B. L.; López-Ribot, J. L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479.
Prasad, T.; Hameed, S.; Manoharlal, R.; Biswas, S.; Mukhopadhyay, C. K.; Goswami, S. K.; Prasad, R. Morphogenic regulator EFG1 affects the drug susceptibilities of pathogenic Candida albicans. FEMS Yeast Res. 2010, 10, 587–596.
Solis, N. V.; Filler, S. G. Mouse model of oropharyngeal candidiasis. Nat. Protoc. 2012, 7, 637–642.
Muñoz, J. E.; Rossi, D. C. P.; Ishida, K.; Spadari, C. C.; Melhem, M. S. C.; Garcia, D. M.; Caires, A. C. F.; Taborda, C. P.; Rodrigues, E. G Antifungal activity of the biphosphinic cyclopalladate C7a against Candida albicans yeast forms in vitro and in vivo. Front. Microbiol. 2017, 8, 771.
Rajabi, F.; Pinilla-de Dios, M.; Luque, R. Highly ordered nanomaterial functionalized copper Schiff base framework: Synthesis, characterization, and hydrogen peroxide decomposition performance. Catalysts 2017, 7, 216.
Xu, P. C.; Yu, H. T.; Li, X. X. Functionalized mesoporous silica for microgravimetric sensing of trace chemical vapors. Anal. Chem. 2011, 83, 3448–3454.
Zhang, M.; Zhang, Y.; Helleur, R. Selective adsorption of Ag+ by ion-imprinted O-carboxymethyl chitosan beads grafted with thiourea-glutaraldehyde. Chem. Eng. J. 2015, 264, 56–65.
Xie, X. Z.; Mao, C. Y.; Liu, X. M.; Zhang, Y. Z.; Cui, Z. D.; Yang, X. J.; Yeung, K. W. K.; Pan, H. B.; Chu, P. K.; Wu, S. L. Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl. Mater. Interfaces 2017, 9, 26417–26428.
Wu, Z. G.; Zhou, W.; Deng, W. J.; Xu, C. L.; Cai, Y.; Wang, X. Y. Antibacterial and hemostatic thiol-modified chitosan-immobilized AgNPs composite sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320.
Yang, F.; Wang, B. B.; Su, H.; Zhou, S. J.; Kong, Y. Facilely self-reduced generation of Ag nanowires in the confined reductive siliceous nanopores and its catalytic reduction property. J. Alloys Compd. 2017, 719, 30–41.
Chong, A. S. M.; Zhao, X. S. Functionalization of SBA-15 with APTES and characterization of functionalized materials. J. Phys. Chem. B 2003, 107, 12650–12657.
Mondal, J.; Sreejith, S.; Borah, P.; Zhao, Y. L. One-pot synthesis of antitumor agent PMX 610 by a copper(II)-incorporated mesoporous catalyst. ACS Sustainable Chem. Eng. 2014, 2, 934–941.
Zhang, W. B.; Shi, T. Y.; Ding, G. L.; Punyapitak, D.; Zhu, J. L.; Guo, D.; Zhang, Z. P.; Li, J. Q.; Cao, Y. S. Nanosilica Schiff-base copper(II) complexes with sustainable antimicrobial activity against bacteria and reduced risk of harm to plant and environment. ACS Sustainable Chem. Eng. 2017, 5, 502–509.
Li, H. N.; Liu, C. B.; Dai, B.; Tang, X. H.; Zhang, Z. J.; Xiong, Z. Q.; Liu, X. M. Synthesis, conductivity, and electromagnetic wave absorption properties of chiral poly Schiff bases and their silver complexes. J. Appl. Polym. Sci. 2015, 132, 42498.
Adur, A. J.; Nandini, N.; Shilpashree, M. K.; Ramya, R.; Srinatha, N. Bio-synthesis and antimicrobial activity of silver nanoparticles using anaerobically digested parthenium slurry. J. Photochem. Photobiol. B: Biol. 2018, 183, 30–34.
Narani, A.; Marella, R. K.; Ramudu, P.; Rao, K. S. R.; Burri, D. R. Cu(II) complex heterogenized on SBA-15: A highly efficient and additive-free solid catalyst for the homocoupling of alkynes. RSC Adv. 2014, 4, 3774–3781.
Parambadath, S.; Mathew, A.; Barnabas, M. J.; Kim, S. Y.; Ha, C. S. Concentration-dependant selective removal of Cr(III), Pb(II) and Zn(II) from aqueous mixtures using 5-methyl-2-thiophenecarboxaldehyde Schiff base-immobilised SBA-15. J. Sol-Gel Sci. Technol. 2016, 79, 426–439.
Roux, S.; Garcia, B.; Bridot, J. L.; Salomé, M.; Marquette, C.; Lemelle, L.; Gillet, P.; Blum, L.; Perriat, P.; Tillement, O. Synthesis, characterization of dihydrolipoic acid capped gold nanoparticles, and functionalization by the electroluminescent luminol. Langmuir 2005, 21, 2526–2536.
Tao, Y. H.; Chen, X. Y.; Jia, F.; Wang, S. X.; Xiao, C. S.; Cui, F. C.; Li, Y. Q.; Bian, Z.; Chen, X. S.; Wang, X. H. New chemosynthetic route to linear e-poly-lysine. Chem. Sci. 2015, 6, 6385–6391.
Cecilia Mesa-Arango, A.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Carlos Argüeelles, J.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of amphotericin b against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638.
Belenky, P.; Camacho, D.; Collins, J. J. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013, 3, 350–358.
Hwang, I. S.; Lee, J.; Hwang, J. H.; Kim, K. J.; Lee, D. G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338.
Purkait, B.; Singh, R.; Wasnik, K.; Das, S.; Kumar, A.; Paine, M.; Dikhit, M.; Singh, D.; Sardar, A. H.; Ghosh, A. K. et al. Up-regulation of silent information regulator 2 (Sir2) is associated with amphotericin b resistance in clinical isolates of Leishmania donovani. J. Antimicrob. Chemother. 2015, 70, 1343–1356.
Guirao-Abad, J. P.; Sánchez-Fresneda, R.; Alburquerque, B.; Hernández, J. A.; Argüelles, J. C. ROS formation is a differential contributory factor to the fungicidal action of amphotericin B and micafungin in Candida albicans. Int. J. Med. Microbiol. 2017, 307, 241–248.
Acknowledgements
This work was supported by the National Key R&D Programs of China (No. 2018YFC0311003 to H. B.), the National Natural Science Foundation of China (No. U1703118 to J. C.), the Natural Science Foundation of Jiangsu Province (No. BK20181364 to J. C.), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, to J. C.), the Cooperative Project between Southeast University and Nanjing Medical University (No. 2018DN0004 to J. C.), the National Science Foundation of the Jiangsu Higher Education Institutions of China (No. 18KJA310002 to H. B., No. 19KJA310003 to J. C.), the Jiangsu Specially Appointed Professor and Jiangsu Medical Specialist Programs of China (to H. B.), and Jiangsu Province “Innovative and Entrepreneurial Team” Program.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2020_3279_MOESM1_ESM.pdf
Schiff-base silver nanocomplexes formation on natural biopolymer coated mesoporous silica contributed to the improved curative effect on infectious microbes
Rights and permissions
About this article
Cite this article
Cai, L., Huang, Y., Duan, Y. et al. Schiff-base silver nanocomplexes formation on natural biopolymer coated mesoporous silica contributed to the improved curative effect on infectious microbes. Nano Res. 14, 2735–2748 (2021). https://doi.org/10.1007/s12274-020-3279-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3279-6