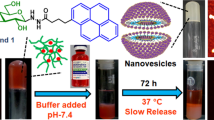
Abstract
Engineered stimuli-responsive drug delivery devices hold vast promise in biological applications for disease treatment due to their maximized therapeutic efficacy. In this study, a novel, stably cross-linked, and pH-sensitive biodegradable gel-micelle was constructed with amphiphilic conjugates of trimethylene dipiperidine-methacrylic anhydride-hyaluronic acid-stearylamine (TMDP-MA-HA-SA, TMHS) to improve tumor-targeting with flexible intracellular delivery of paclitaxel (PTX).The cross-linked methacrylate bonds significantly improved the biostability of TMHS gel-micelle (~ 200 nm) over the non-cross-linked under physiological conditions, while hyaluronic acid plays an important role in active tumor targetability. The gradual degradation of cross-linked hyaluronic acid shell was triggered by the concentrated hyaluronidase. Meanwhile, under acidic conditions (pH < 6.5), the tertiary amines of pH-sensitive TMDP moieties were protonated and thereby solubilized the gel-micellar core-portions. The resultant pH-triggered inner-core spaces rapidly prompted PTX release in the presence of multiple cytosolic enzymes that mainly degraded the remaining hydrophobic stearylamine core. During the in vitro cytotoxicity assay, PTX-loaded TMHS gel-micelles (CLTMHSPTX) revealed anticancer efficacy against human hepatocellular carcinoma HepG2 cells with IC50 of 1.42 μg/mL (PTX concentration), significantly lower than other groups. In parallel, the in vivo anti-tumor efficacy of CLTMHSPTX gel-micelles against BALB/c xenograft tumor animal model demonstrated the greater tumor growth inhibition capacity of 72.06%, compared to other treatment groups at a safe concentration. Consequently, the cross-linked and stimuli-responsive CLTMHSPTX gel-micelles hold a great potential for flexible modulation of intracellular delivery of hydrophobic anticancer drugs with maximized antitumor efficacy.

Similar content being viewed by others
References
Kuang, H. H.; Ku, S. H.; Kokkoli, E. The design of peptide-amphiphiles as functional ligands for liposomal anticancer drug and gene delivery. Adv. Drug Deliver. Rev. 2016, 110–111, 80–101.
Jian, C.; Xin, T.; Jie, Z.; Shi, T.; Peng, Z.; Chao, L. Multifunctional cationic polyurethanes designed for non-viral cancer gene therapy. Acta Biomater. 2016, 30, 155–167.
Sahu, P.; Kashaw, S. K.; Jain, S.; Sau, S.; Iyer, A. K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J. Control. Release 2017, 253, 122–136.
Stocke, N. A.; Sethi, P.; Jyoti, A.; Chan, R.; Arnold, S. M.; Hilt, J. Z.; Upreti, M. Toxicity evaluation of magnetic hyperthermia induced by remote actuation of magnetic nanoparticles in 3D micrometastasic tumor tissue analogs for triple negative breast cancer. Biomaterials 2017, 120, 115–125.
Liu, Y.; Wan, G. Y.; Guo, H.; Liu, Y. Y.; Zhou, P.; Wang, H. M.; Wang, D.; Zhang, S. P.; Wang, Y. S.; Zhang, N. A multifunctional nanoparticle system combines sonodynamic therapy and chemotherapy to treat hepatocellular carcinoma. Nano Res. 2017, 10, 834–855.
Chen, Y.; Li, H. H.; Deng, Y. Y.; Sun, H. F.; Xue, K.; Ci, T. Y. Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment. Acta Biomater. 2017, 51, 374–392.
Cirillo, G.; Spizzirri, U. G.; Curcio, M.; Hampel, S.; Vittorio, O.; Restuccia, D.; Picci, N.; Iemma, F. Carbon nanohybrids as electro-responsive drug delivery systems. Mini Rev. Med. Chem. 2016, 16, 658–667.
Li, T. S.; Amari, T.; Semba, K.; Yamamoto, T.; Takeoka, S. Construction and evaluation of pH-sensitive immunoliposomes for enhanced delivery of anticancer drug to ErbB2 over-expressing breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1219–1227.
Meng, H.; Wang, M. Y.; Liu, H. Y.; Liu, X. S.; Situ, A.; Wu, B.; Ji, Z. X.; Chang, C. H.; Nel, A. E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557.
Liao, J. W.; Liu, P. P.; Hou, G. X.; Shao, J. J.; Jing, Y.; Liu, K. Y.; Lu, W. H.; Wen, S. J.; Hu, Y. M.; Peng, H. Regulation of stem-like cancer cells by glutamine through β-catenin pathway mediated by redox signaling. Mol. Cancer. 2017, 16, 51.
Harnoy, A. J.; Rosenbaum, I.; Tirosh, E.; Ebenstein, Y.; Shaharabani, R.; Beck, R.; Amir, R. J. Enzyme-responsive amphiphilic PEG-dendron hybrids and their assembly into smart micellar nanocarriers. J. Am. Chem. Soc. 2014, 136, 7531–7534.
Davaa, E.; Lee, J.; Jenjob, R.; Yang, S. G. Mt1-mmp responsive doxorubicin conjugated poly (lactic-co-glycolic acid)/poly (styrene- alt-maleic anhydride) core/shell microparticles for intrahepatic arterial chemotherapy of hepatic cancer. ACS Appl. Mater. Interfaces 2017, 9, 71–79.
Chen, W. H.; Luo, G. F.; Lei, Q.; Hong, S.; Qiu, W. X.; Liu, L. H.; Cheng, S. X.; Zhang, X. Z. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano 2017, 11, 1419–1431.
Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640.
Liang, X. L.; Fang, L.; Li, X. D.; Zhang, X.; Wang, F. Activatable near infrared dye conjugated hyaluronic acid based nanoparticles as a targeted theranostic agent for enhanced fluorescence/CT/photoacoustic imaging guided photothermal therapy. Biomaterials 2017, 132, 72–84.
Cai, Y. P.; López-Ruiz, E.; Wengel, J.; Creemers, L. B.; Howard, K. A. A hyaluronic acid-based hydrogel enabling CD44-mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. J. Control. Release 2017, 253, 153–159.
Zhou, B.; Weigel, J. A.; Fauss, L.; Weigel, P. H. Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2000, 275, 37733–37741.
Yang, C. C.; Li, C.; Zhang, P.; Wu, W.; Jiang, X. Q. Redox responsive hyaluronic acid nanogels for treating rhamm (CD168) over-expressive cancer, both primary and metastatic tumors. Theranostics 2017, 7, 1719–1734.
Wickens, J. M.; Alsaab, H. O.; Kesharwani, P.; Bhise, K.; Amin, M. C. I. M.; Tekade, R. K.; Gupta, U.; Iyer, A. K. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680.
Jeong, J. Y.; Hong, E. H.; Lee, S. Y.; Lee, J. Y.; Song, J. H.; Ko, S. H.; Shim, J. S.; Choe, S.; Kim, D. D.; Ko, H. J. et al. Boronic acid-tethered amphiphilic hyaluronic acid derivative-based nanoassemblies for tumor targeting and penetration. Acta Biomater. 2017, 53, 414–426.
Zhu, D. Q.; Wang, H. Y.; Trinh, P.; Heilshorn, S. C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140.
Noh, I.; Kim, H. O.; Choi, J.; Choi, Y.; Dong, K. L.; Huh, Y. M.; Haam, S. Co-delivery of paclitaxel and gemcitabine via CD44-targeting nanocarriers as a prodrug with synergistic antitumor activity against human biliary cancer. Biomaterials 2015, 53, 763–774.
Han, J.; Park, W.; Park, S.; Na, K. Photosensitizer-conjugated hyaluronic acid-shielded polydopamine nanoparticles for targeted photo-mediated tumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 7739–7747.
Deng, C.; Jiang, Y. J.; Cheng, R.; Meng, F. H.; Zhong, Z. Y. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480.
Brugués, A. P.; Naveros, B. C.; Calpena Campmany, A. C.; Pastor, P. H.; Saladrigas, R. F.; Lizandra, C. R. Developing cutaneous applications of paromomycin entrapped in stimuli- sensitive block copolymer nanogel dispersions. Nanomedicine 2015, 10, 227–240.
Tang, L. M.; Zhou, M. L.; Huang, Y.; Zhong, J. J.; Zhou, Z.; Luo, K. Dual-sensitive and biodegradable core-crosslinked HPMA copolymer-doxorubicin conjugate-based nanoparticles for cancer therapy. Polymer Chem. 2017, 8, 2370–2380.
Zhou, Z. W.; Li, H. P.; Wang, K. K.; Guo, Q.; Li, C. Z.; Jiang, H. L.; Hu, Y. Q.; Oupicky, D.; Sun, M. J. Bioreducible cross-linked hyaluronic acid/calcium phosphate hybrid nanoparticles for specific delivery of siRNA in melanoma tumor therapy. ACS Appl. Mater. Interfaces 2017, 9, 14576–14589.
Yang, C. C.; Wang, X.; Yao, X. K.; Zhang, Y. J.; Wu, W.; Jiang, X. Q. Hyaluronic acid nanogels with enzyme-sensitive cross- linking group for drug delivery. J. Control. Release 2015, 205, 206–217.
Guan, X. W.; Li, Y. H.; Jiao, Z. X.; Chen, J.; Guo, Z. P.; Tian, H. Y.; Chen, X. S. A pH-sensitive charge-conversion system for doxorubicin delivery. Acta Biomater. 2013, 9, 7672–7678.
Wang, D. G.; Wang, T. T.; Liu, J. P.; Yu, H. J.; Shi, J.; Bing, F.; Zhou, F. Y.; Fu, Y. L.; Yin, Q.; Zhang, P. C. et al. Acid-activatable versatile micelleplexes for PD-L1 blockade- enhanced cancer photodynamic immunotherapy. Nano Lett. 2016, 16, 5503–5513.
Liu, J.; Huang, Y. R.; Kumar, A.; Tan, A.; Jin, S. B.; Mozhi, A.; Liang, X. J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710.
Ma, J.; Kang, K.; Yi, Q. Y.; Zhang, Z. R.; Gu, Z. W. Multiple pH responsive zwitterionic micelles for stealth delivery of anticancer drugs. RSC Adv. 2016, 6, 64778–64790.
Cong, T. H.; Kang, S. W.; Li, Y.; Kim, B. S.; Lee, D. S. Controlled release of human growth hormone from a biodegradable pH/temperature-sensitive hydrogel system. Soft Matter 2011, 7, 8984–8990.
Liu, Y. H.; Sun, J.; Cao, W.; Yang, J. H.; Lian, H.; Li, X.; Sun, Y. H.; Wang, Y. J.; Wang, S. L.; He, Z. G. Dual targeting folate- conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int. J. Pharmaceutics 2011, 421, 160–169.
Hachet, E.; Van Den Berghe, H.; Bayma, E.; Block, M.; Auzély-Velty, R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules 2012, 13, 1818–1827.
Cui, C.; Xue, Y. N.; Wu, M.; Zhang, Y.; Yu, P.; Liu, L.; Zhuo, R. X.; Huang, S. W. Cellular uptake, intracellular trafficking, and antitumor efficacy of doxorubicin-loaded reduction-sensitive micelles. Biomaterials 2013, 34, 3858–3869.
Jiang, Y.; Wang, X. Z.; Liu, X.; Lv, W.; Zhang, H. J.; Zhang, M. W.; Li, X. R.; Xin, H. L.; Xu, Q. W. Enhanced antiglioma efficacy of ultrahigh loading capacity paclitaxel prodrug conjugate self-assembled targeted nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 211–217.
Cho, E. J.; Sun, B.; Doh, K. O.; Wilson, E. M.; Torregrosa-Allen, S.; Elzey, B. D.; Yeo, Y. Intraperitoneal delivery of platinum with in-situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer. Biomaterials 2015, 37, 312–319.
Ding, X. F.; Wang, W.; Wang, Y. Z.; Bao, X. L.; Wang, Y.; Wang, C.; Chen, J.; Zhang, F. R.; Zhou, J. P. Versatile reticular polyethylenimine derivative-mediated targeted drug and gene codelivery for tumor therapy. Mol. Pharmaceutics 2014, 11, 3307–3321.
Han, S.; Liu, Y.; Nie, X.; Xu, Q.; Jiao, F.; Li, W.; Zhao, Y.; Wu, Y.; Chen, C. Efficient delivery of antitumor drug to the nuclei of tumor cells by amphiphilic biodegradable poly(L-aspartic acid- co-lactic acid)/DPPE co-polymer nanoparticles. Small 2012, 8, 1596–1606.
Yue, J.; Liu, S.; Wang, R.; Hu, X. L.; Xie, Z. G.; Huang, Y. B.; Jing, X. B. Transferrin-conjugated micelles: Enhanced accumulation and antitumor effect for transferrin-receptor-overexpressing cancer models. Mol. Pharmaceutics 2012, 9, 1919–1931.
Raemdonck, K.; Martens, T. F.; Braeckmans, K.; Demeester, J.; De Smedt, S. C. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliver. Rev. 2013, 65, 1123–1147.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Aouameur, D., Cheng, H., Opoku-Damoah, Y. et al. Stimuli-responsive gel-micelles with flexible modulation of drug release for maximized antitumor efficacy. Nano Res. 11, 4245–4264 (2018). https://doi.org/10.1007/s12274-018-2012-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2012-1