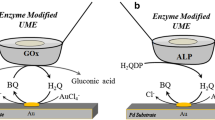
Abstract
Scanning electrochemical microscopy represents a powerful tool for electro(chemical) characterization of surfaces, but its applicability has been limited in most cases at microscale spatial resolution, and the greatest challenge has been the scaling down to the nanoscale for fabrication and the use of nanometer-sized tips. Here, Pt nanoelectrodes with nanometer electroactive area were fabricated and employed for imaging a distribution of gold nanoparticles (AuNPs) and bioelectrocatalytic activity of a redox-active enzyme immobilized on gold surfaces.

Similar content being viewed by others
References
Bard, A. J.; Fan, F. R. F.; Kwak, J.; Lev, O. Scanning electrochemical microscopy. Introduction and principles. Anal. Chem. 1989, 61, 132–138.
Scanning Electrochemical Microscopy; Mirkin, M. V.; Bard, A. J., Eds.; Marcel Dekker: New York, 2001.
Wittstock, G.; Burchardt, M.; Pust, S. E.; Shen, Y.; Zhao, C. Scanning electrochemical microscopy for direct imaging of reaction rates. Angew. Chem., Int. Ed. 2007, 46, 1584–1617.
Takahashi, Y.; Hirano, Y.; Yasukawa, T.; Shiku, H.; Yamada, H.; Matsue, T. Topographic, electrochemical, and optical images captured using standing approach mode scanning electrochemical/optical microscopy. Langmuir 2006, 22, 10299–10306.
Tan, C.; Rodríguez-López, J.; Parks, J. J.; Ritzert, N. L.; Ralph, D. C.; Abruña, H. D. Reactivity of monolayer chemical vapor deposited graphene imperfections studied using scanning electrochemical microscopy. ACS Nano 2012, 6, 3070–3079.
Rodríguez-López, J.; Ritzert, N. L.; Mann, J. A.; Tan, C.; Dichtel, W. R.; Abruña, H. D. Quantification of the surface diffusion of tripodal binding motifs on graphene using scanning electrochemical microscopy. J. Am. Chem. Soc. 2012, 134, 6224–6236.
Roberts, W. S.; Lonsdale, D. J.; Griffiths, J.; Higson, S. P. J. Advances in the application of scanning electrochemical microscopy to bioanalytical systems. Biosens. Bioelectron. 2007, 23, 301–318.
Parra, A.; Casero, E.; Vázquez, L.; Jin, J.; Pariente, F.; Lorenzo, E. Microscopic and voltammetric characterization of bioanalytical platforms based on lactate oxidase. Langmuir 2006, 22, 5443–5450.
Horrocks, B. R.; Wittstock, G. Biotechnological applications. In Scanning Electrochemical Microscopy; Mirkin, M. V.; Bard, A. J., Eds.; CRC Press: Boca Raton, 2012; pp 318–370.
Edwards, M. A.; Martin, S.; Whitworth, A. L.; Macpherson, J. V.; Unwin, P. R. Scanning electrochemical microscopy: Principles and applications to biophysical systems. Physiol. Measure. 2006, 27, R63–R108.
Pierce, D. T.; Unwin, P. R.; Bard, A. J. Scanning electrochemical microscopy. 17. Studies of enzyme-mediator kinetics for membrane- and surface-immobilized glucose oxidase. Anal. Chem. 1992, 64, 1795–1804.
Pellissier, M.; Zigah, D.; Barrière, F.; Hapiot, P. Optimized preparation and scanning electrochemical microscopy analysis in feedback mode of glucose oxidase layers grafted onto conducting carbon surfaces. Langmuir 2008, 24, 9089–9095.
Nogala, W.; Szot, K.; Burchardt, M.; Roelfs, F.; Rogalski, J.; Opallo, M.; Wittstock, G. Feedback mode SECM study of laccase and bilirubin oxidase immobilised in a sol-gel processed silicate film. Analyst 2010, 135, 2051–2058.
Zhao, C.; Wittstock, G. Scanning electrochemical microscopy for detection of biosensor and biochip surfaces with immobilized pyrroloquinoline quinone (PQQ)-dependent glucose dehydrogenase as enzyme label. Biosens. Bioelectron. 2005, 20, 1277–1284.
Schäfer, D.; Maciejewska, M.; Schuhmann, W. SECM visualization of spatial variability of enzyme–polymer spots: 1. Discretisation and interference elimination using artificial neural networks. Biosens. Bioelectron. 2007, 22, 1887–1895.
Zhao, C.; Wittstock, G. Scanning electrochemical microscopy of quinoprotein glucose dehydrogenase. Anal. Chem. 2004, 76, 3145–3154.
Wittstock, G.; Schuhmann, W. Formation and imaging of microscopic enzymatically active spots on an alkanethiolatecovered gold electrode by scanning electrochemical microscopy. Anal. Chem. 1997, 69, 5059–5066.
Zhao, C.; Sinha, J. K.; Wijayawardhana, C. A.; Wittstock, G. Monitoring ß-galactosidase activity by means of scanning electrochemical microscopy. J. Electroanal. Chem. 2004, 561, 83–91.
Arrigan, D. W. M. Nanoelectrodes, nanoelectrode arrays and their applications. Analyst 2004, 129, 1157–1165.
Katemann, B. B.; Schuhmann, W. Fabrication and characterization of needle-type. Electroanalysis 2002, 14, 22–28.
Shao, Y. H.; Mirkin, M. V.; Fish, G.; Kokotov, S.; Palanker, D.; Lewis, A. Nanometer-sized electrochemical sensors. Anal. Chem. 1997, 69, 1627–1634.
Murray, R. W. Nanoelectrochemistry: Metal nanoparticles, nanoelectrodes, and nanopores. Chem. Rev. 2008, 108, 2688–2720.
Mirkin, M. V.; Fan, F.-R. F.; Bard, A. J. Scanning electrochemical microscopy part 13. Evaluation of the tip shapes of nanometer size microelectrodes. J. Electroanal. Chem. 1992, 328, 47–62.
Melmed, A. J. The art and science and other aspects of making sharp tips. J. Vacuum Sci. Technol. B: Microelectron. Nanometer Struct. Process. Measure. Phenom. 1991, 9, 601–608.
Bach, C. E.; Nichols, R. J.; Beckmann, W.; Meyer, H.; Schulte, A.; Besenhard, J. O.; Jannakoudakis, P. D. Effective insulation of scanning tunneling microscopy tips for electrochemical studies using an electropainting method. J. Electrochem. Soc. 1993, 140, 1281–1284.
Slevin, C. J.; Gray, N. J.; Macpherson, J. V.; Webb, M. A.; Unwin, P. R. Fabrication and characterisation of nanometresized platinum electrodes for voltammetric analysis and imaging. Electrochem. Commun. 1999, 1, 282–288.
Zhang, B.; Galusha, J.; Shiozawa, P. G.; Wang, G. L.; Bergren, A. J.; Jones, R. M.; White, R. J.; Ervin, E. N.; Cauley, C. C.; White, H. S. Bench-top method for fabricating glass-sealed nanodisk electrodes, glass nanopore electrodes, and glass nanopore membranes of controlled size. Anal. Chem. 2007, 79, 4778–4787.
Macpherson, J. V.; Unwin, P. R. Combined scanning electrochemical–atomic force microscopy. Anal. Chem. 2000, 72, 276–285.
Li, Y. X.; Bergman, D.; Zhang, B. Preparation and electrochemical response of 1–3 nm Pt disk electrodes. Anal. Chem. 2009, 81, 5496–5502.
Watkins, J. J.; Chen, J. Y.; White, H. S.; Abruña, H. D.; Maisonhaute, E.; Amatore, C. Zeptomole voltammetric detection and electron-transfer rate measurements using platinum electrodes of nanometer dimensions. Anal. Chem. 2003, 75, 3962–3971.
Pendley, B. D.; Abruna, H. D. Construction of submicrometer voltammetric electrodes. Anal. Chem. 1990, 62, 782–784.
Agyekum, I.; Nimley, C.; Yang, C. X.; Sun, P. Combination of scanning electron microscopy in the characterization of a nanometer-sized electrode and current fluctuation observed at a nanometer-sized electrode. J. Phys. Chem. C 2010, 114, 14970–14974.
Baltes, N.; Thouin, L.; Amatore, C.; Heinze, J. Imaging concentration profiles of redox-active species with nanometric amperometric probes: Effect of natural convection on transport at microdisk electrodes. Angew. Chem., Int. Ed. 2004, 43, 1431–1435.
Schulte, A.; Chow, R. H. A simple method for insulating carbon-fiber microelectrodes using anodic electrophoretic deposition of paint. Anal. Chem. 1996, 68, 3054–3058.
Conyers, J. L.; White, H. S. Electrochemical characterization of electrodes with submicrometer dimensions. Anal. Chem. 2000, 72, 4441–4446.
Watkins, J. J.; White, H. S. The role of the electrical double layer and ion pairing on the electrochemical oxidation of hexachloroiridate(III) at Pt electrodes of nanometer dimensions. Langmuir 2004, 20, 5474–5483.
Sun, P.; Zhang, Z. Q.; Guo, J. D.; Shao, Y. H. Fabrication of nanometer-sized electrodes and tips for scanning electrochemical microscopy. Anal. Chem. 2001, 73, 5346–5351.
Liu, B.; Rolland, J. P.; DeSimone, J. M.; Bard, A. J. Fabrication of ultramicroelectrodes using a “Teflon-like” coating material. Anal. Chem. 2005, 77, 3013–3017.
Fan, F.-R. F.; Bard, A. J. Electrochemical detection of single molecules. Science 1995, 267, 871–874.
Fan, F.-R. F.; Kwak, J.; Bard, A. J. Single molecule electrochemistry. J. Am. Chem. Soc. 1996, 118, 9669–9675.
Bard, A. J.; Fan, F.-R. F. Electrochemical detection of single molecules. Acc. Chem. Res. 1996, 29, 572–578.
Penner, R. M.; Heben, M. J.; Longin, T. L.; Lewis, N. S. Fabrication and use of nanometer-sized electrodes in electrochemistry. Science 1990, 250, 1118–1121.
Hrapovic, S.; Luong, J. H. T. Picoamperometric detection of glucose at ultrasmall platinum-based biosensors: Preparation and characterization. Anal. Chem. 2003, 75, 3308–3315.
Tel-Vered, R.; Bard, A. J. Generation and detection of single metal nanoparticles using scanning electrochemical microscopy techniques. J. Phys. Chem. B 2006, 110, 25279–25287.
Lai, S. C. S.; Dudin, P. V.; Macpherson, J. V.; Unwin, P. R. Visualizing zeptomole (electro)catalysis at single nanoparticles within an ensemble. J. Am. Chem. Soc. 2011, 133, 10744–10747.
Kim, J.; Renault, C.; Nioradze, N.; Arroyo-Currás, N.; Leonard, K. C.; Bard, A. J. Electrocatalytic activity of individual Pt nanoparticles studied by nanoscale scanning electrochemical microscopy.. J. Am. Chem. Soc. 2016, 138, 8560–8568.
Abad, J. M.; Tesio, A. Y.; Pariente, F.; Lorenzo, E. Patterning gold nanoparticle using scanning electrochemical microscopy. J. Phys. Chem. C 2013, 117, 22087–22093
Ferreira, M.; Varela, H.; Torresi, R. M.; Tremiliosi-Filho, G. Electrode passivation caused by polymerization of different phenolic compounds. Electrochim. Acta 2006, 52, 434–442.
Llopis, J. F.; Colom; F. Encyclopedia of electrochemistry of the elements. In Encyclopedia of Electrochemistry of the Elements; Bard, A. J., Ed.; Marcel Dekker: New York, 1976; Vol. 6, pp 224–226.
Haiss, W.; Martín, S.; Leary, E.; van Zalinge, H.; Higgins, S. J.; Bouffier, L.; Nichols, R. J. Impact of junction formation method and surface roughness on single molecule conductance. J. Phys. Chem. C 2009, 113, 5823–5833.
Brust, M.; Bethell, D.; Kiely, C. J.; Schiffrin, D. J. Selfassembled gold nanoparticle thin films with nonmetallic optical and electronic properties. Langmuir 1998, 14, 5425–5429.
Love, J. C.; Estroff, L. A.; Kriebel, J. K.; Nuzzo, R. G.; Whitesides, G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170.
Aizenberg, J.; Black, A. J.; Whitesides, G. M. Controlling local disorder in self-assembled monolayers by patterning the topography of their metallic supports. Nature 1998, 394, 868–871.
Madoz-Gúrpide, J.; Abad, J. M.; Fernández-Recio, J.; Vélez, M.; Vázquez, L.; Gómez-Moreno, C.; Fernández, V. M. Modulation of electroenzymatic NADPH oxidation through oriented immobilization of ferredoxin:NADP+reductase onto modified gold electrodes. J. Am. Chem. Soc. 2000, 122, 9808–9817.
Darder, M.; Takada, K.; Pariente, F.; Lorenzo, E.; Abruña, H. D. Dithiobissuccinimidyl propionate as an anchor for assembling peroxidases at electrodes surfaces and its application in a H2O2 biosensor. Anal. Chem. 1999, 71, 5530–5537.
Leiros, I.; Wang, E.; Rasmussen, T.; Oksanen, E.; Repo, H.; Petersen, S. B.; Heikinheimo, P.; Hough, E. The 2.1 Å structure of Aerococcus viridans l-lactate oxidase (LOX). Acta Cryst. 2006, 62, 1185–1190.
Parra, A.; Casero, E.; Vázquez, L.; Pariente, F.; Lorenzo, E. Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal. Chim. Acta 2006, 555, 308–315.
Acknowledgements
J. M. A. acknowledges research funding by a “Ramon y Cajal” contract from the Spanish Ministry of Science and Innovation. A. Y. T. acknowledges a fellowship from CONICET and Fundación Carolina. We are grateful to Prof. Luis Vázquez and Tech. Andrés Valera (ICMM-CSIC) for carrying out AFM and SEM measurements, respectively. The authors also thank Dr. Elena Casero (UAM) for support in the use of SECM instrumentation and Prof. H. D. Abruña for critically reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Abad, J.M., Tesio, A.Y., Martínez-Periñán, E. et al. Imaging resolution of biocatalytic activity using nanoscale scanning electrochemical microscopy. Nano Res. 11, 4232–4244 (2018). https://doi.org/10.1007/s12274-018-2011-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2011-2