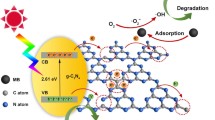
Abstract
A template-free hydrothermal-assisted thermal polymerization method has been developed for the large-scale synthesis of one-dimensional (1D) graphitic carbonnitride (g-C3N4) microtubes. The g-C3N4 microtubes were obtained by simple thermal polymerization of melamine-cyanuric acid complex microrods under N2 atmosphere, which were synthesized by hydrothermal treatment of melamine solution at 180 °C for 24 h. The as-obtained g-C3N4microtubes exhibited a large surface area and a unique one-dimensional tubular structure, which provided abundant active sites for proton reduction and also facilitated the electron transfer processes. As such, the g-C3N4 microtubes showed enhanced photocatalytic H2 productionactivity in lactic acid aqueous solutions under visible light irradiation (λ ≥ 420 nm), which was ∼ 3.1 times higher than that of bulk g-C3N4 prepared by direct thermal polymerization of the melamine precursor under the same calcination conditions.

Similar content being viewed by others
References
Liu, J.; Liu, Y; Liu, N. Y; Han, Y. Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S. T.; Zhong, J.; Kang, Z. H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974.
Wang, X. C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G; Carlsson J. M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80.
Yu, H. J.; Shi, R.; Zhao, Y X.; Bian, T.; Zhao, Y. F.; Zhou, C.; Waterhouse, G. I. N.; Wu, L.-Z. Tung, C.-H.; Zhang, T. R. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148.
Zhu, M. S.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J. Y; Wang, X. C.; Majima, T. Metal-free photocatalyst for H2 evolution in visible to near-infrared region: Black phosphorus/graphitic carbon nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242.
Zhang, G G.; Li, G. S.; Lan, Z. A.; Lin, L. H.; Savateev, A.; Heil, T.; Zafeiratos, S.; Wang, X. C.; Antonietti, M. Optimizing optical absorption, exciton dissociation, and charge transfer of a polymeric carbon nitride with ultrahigh solar hydrogen production activity. Angew. Chem, Int. Ed 2017, 56, 13445–13449.
Liu, C. Y; Huang, H. W.; Ye, L. Q.; Yu, S. X.; Tian, N.; Du, X.; Zhang, T. R.; Zhang, Y. H. Intermediate-mediated strategy to horn-like hollow mesoporous ultrathin g-C3N4 tube with spatial anisotropic charge separation for superior photocatalytic H2 evolution. Nano Energy 2017, 41, 738–748.
Li, C. M.; Du, Y H.; Wang, D. P.; Yin, S. M.; Tu, W. G.; Chen, Z.; Kraft, M.; Chen, G; Xu, R. Unique P-Co-N surface bonding states constructed on g-C3N4 nanosheets for drastically enhanced photocatalytic activity of H2 evolution. Adv. Funct. Mater. 2017, 27, 1604328.
Niu, P.; Yin, L. C.; Yang, Y Q.; Liu, G.; Cheng, H. M. Increasing the visible light absorption of graphitic carbon nitride (Melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Adv. Mater. 2014, 26, 8046–8052.
Bai, S.; Wang, X. J.; Hu, C. Y.; Xie, M. L.; Jiang, J.; Xiong, Y. J. Two-dimensional g-C3N4: An ideal platform for examining facet selectivity of metal co-catalysts in photocatalysis. Chem. Commun. 2014, 50, 6094–6096.
Chen, X. F.; Zhang, J. S.; Fu, X. Z.; Antonietti, M.; Wang, X. C. Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Am. Chem. Soc. 2009, 131, 11658–11659.
Niu, P.; Zhang, L. L.; Liu, G; Chen, H. M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770.
Yang, S. B.; Gong, Y. J.; Zhang, J. S.; Zhan, L.; Ma, L. L.; Fang, Z. Y; Vajtai, R.; Wang, X. C.; Ajayan, P. M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456.
Zhao, Y; Zhao, F.; Wang, X. P.; Xu, C. Y.; Zhang, Z. P.; Shi, G. Q.; Qu, L. T. Graphitic carbon nitride nanoribbons: Grapheneassisted formation and synergic function for highly efficient hydrogen evolution. Angew. Chem., Int. Ed. 2014, 53, 1393413939.
Sun, J. H.; Zhang, J. S.; Zhang, M. W.; Antonietti, M.; Fu, X. Z.; Wang, X. C. Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun. 2012, 3, 1139.
Zhang, Y. H.; Pan, Q. W.; Chai, G. Q.; Liang, M. R.; Dong, G. P.; Zhang, Q. Y.; Qiu, J. R. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943.
Bai, X. J.; Wang, L.; Zong, R. L.; Zhu, Y F. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961.
Tahir, M.; Cao, C. B.; Mahmood, N.; Butt, F. K.; Mahmood, A.; Idrees, F.; Hussain, S.; Tanveer, M.; Ali, Z.; Aslam, I. Multifunctional g-C3N4 nanofibers: A template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACSAppl. Mater. Interfaces 2014, 6, 1258–1265.
Li, J.; Cao, C. B.; Zhu, H. S. Synthesis and characterization of graphite-like carbon nitride nanobelts and nanotubes. Nanotechnology 2007, 18, 115605.
Guo, Q. X.; Xie, Y; Wang, X. J.; Zhang, S. Y; Hou. T.; Lv, S. C. Synthesis of carbon nitride nanotubes with the C3N4 stoichiometry via a benzene-thermal process at low temperatures. Chem. Commun. 2004, 26–27.
Wang, S. P.; Li, C. J.; Wang, T.; Zhang, P.; Li, A.; Gong, J. L. Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J Mater. Chem. A 2014, 2, 2885–2890.
Tahir, M.; Cao, C. B.; Butt, F. K.; Idrees, F.; Mahmood, N.; Ali, Z.; Aslam, I.; Tanveer, M.; Rizwan, M.; Mahood, T. Tubular graphitic-C3N4: A prospective material for energy storage and green photocatalysis. J. Mater. Chem. A 2013, 1, 13949–13955.
Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 93859454.
Zhang, H. J.; Zuo, X. Q.; Tang, H. B.; Li, G; Zhou, Z. Origin of photoactivity in graphitic carbon nitride and strategies for enhancement of photocatalytic efficiency: Insights from firstprinciples computations. Phys. Chem. Chem. Phys. 2015, 17, 62806288.
Gracia, J.; Kroll, P. First principles study of C3N4 carbon nitride nanotubes. J. Mater. Chem. 2009, 19, 3020–3026.
Chai, G. L.; Lin, C. S.; Wei, J.; Zhang, M. Y; Cheng, W. D. Nonlinear optical properties of carbon nitride nanotubes. Phys. Chem. Chem. Phys. 2012, 14, 835–839.
Pan, H.; Zhang, Y W.; Shenoy, V. B.; Gao, H. J. Ab initio study on a novel photocatalyst: Functionalized graphitic carbon nitride nanotube. ACS Catal. 2011, 1, 99–104.
Gao, J.; Zhou, Y; Li, Z. S.; Yan, S. C.; Wang, N. Y.; Zou, Z. G. High-yield synthesis of millimetre-long, semiconducting carbon nitride nanotubes with intense photoluminescence emission and reproducible photoconductivity. Nanoscale 2012, 4, 3687–3692.
Cao, C. B.; Huang, F. L.; Cao, C. T.; Li, J.; Zhu, H. S. Synthesis of carbon nitride nanotubes via a catalytic-assembly solvothermal route. Chem. Mater. 2004, 16, 5213–5215.
Jun, Y. S.; Lee, E. Z.; Wang, X. C.; Hong, W. H.; Stucky, G. D.; Thomas, A. From melamine-cyanuric acid supramolecular aggregates to carbon nitride hollow spheres. Adv. Funct. Mater. 2013, 23, 3661–3667.
Cao, S. W.; Low, J. X.; Yu, J. G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176.
Zhang, G. G; Zang, S. H.; Wang, X. C. Layered Co(OH)2 deposited polymeric carbon nitrides for photocatalytic water oxidation. ACS Catal. 2015, 5, 941–947.
Ong, W. J.; Putri, L. K.; Tan, Y C.; Tan, L. L.; Li, N.; Ng, Y. H.; Wen, X. M.; Chai, S. P. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study. Nano Res. 2017, 10, 1673–1696.
Shalom, M.; Inal, S.; Fettkenhaure, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121.
Jun, Y. S.; Park, J.; Lee, S. U.; Thomas, A.; Hong, W. H.; Stucky, G. D. Three-dimensional macroscopic assemblies of low-dimensional carbon nitrides for enhanced hydrogen evolution. Angew. Chem, Int. Ed. 2013, 52, 11083–11087.
Guo, S. E.; Deng, Z. P.; Li, M. X.; Jiang, B. J.; Tian, C. G.; Pan, Q. J.; Fu, H. G. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem., Int. Ed. 2016, 55, 18301834.
Zhang, Q.; Joo, J.-B.; Lu, Z. D.; Dahl, M.; Oliveira, D. Q. L.; Ye, M. M.; Yin, Y. D. Self-assembly and photocatalysis of mesoporous TiO2 nanocrystal clusters. Nano Res. 2011, 4, 103114.
Zhou, C.; Zhao, Y. F.; Bian, T.; Shang, L.; Yu, H. J.; Wu, L. Z.; Tung, C. H.; Zhang, T. R. Bubble template synthesis of Sn2Nb2O7 hollow spheres for enhanced visible-light-driven photocatalytic hydrogen production. Chem. Commun. 2013, 49, 9872–9874.
Pan, B.; Zhou, Y. G.; Su, W. Y; Wang, X. X. Self-assembly synthesis of LaPO4 hierarchical hollow spheres with enhanced photocatalytic CO2-reduction performance. Nano Res. 2017, 10, 534–545.
Zheng, Y; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S. Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731.
Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2014CB239402), the National Key Projects for Fundamental Research and Development of China (Nos. 2016YFB0600901, 2017YFA0206904, and 2017YFA0206900), the National Natural Science Foundation of China (Nos. 51772305, 51572270, U1662118, and 21401207), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB17000000), and the Youth Innovation Promotion Association of the CAS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, C., Shi, R., Shang, L. et al. Template-free large-scale synthesis of g-C3N4 microtubes for enhanced visible light-driven photocatalytic H2 production. Nano Res. 11, 3462–3468 (2018). https://doi.org/10.1007/s12274-018-2003-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2003-2