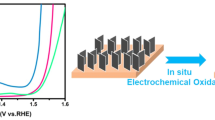
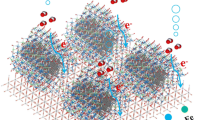
Abstract
The development of new non-precious metal catalysts and understanding the origin of their activity for the hydrogen evolution reaction (HER) are essential for rationally designing highly active low-cost catalysts as alternatives to state-of-the-art precious metal catalysts. Herein, manganese oxide/hydroxide was demonstrated as a highly active electrocatalysts for the HER by fabricating MnO2 nanosheets coated with Cu2O nanowire arrays (Cu2O@MnO2 NW@NS) on Cu foam followed by an in situ chronopotentiometry (CP) treatment. It was discovered that the in situ transformation of Cu2O@MnO2 into Cu@Mn(OH)2 NW@NS by the CP treatment drastically boosted the catalytic activity for the HER due to an enhancement of its intrinsic activity. Together with the benefits from such three-dimensional (3D) core–shell arrays for exposing more accessible active sites and efficient mass and electron transfers, the resulting Cu@Mn(OH)2 NW@NS exhibited excellent HER activity and outstanding durability in terms of a low overpotential of 132 mV vs. RHE at 10 mA/cm2. Overall, we expect these findings to generate new opportunities for the exploration of other Mn-based nanomaterials as efficient electrocatalysts and enable further understanding of their catalytic processes.

Similar content being viewed by others
References
Le Goff, A.; Artero, V.; Jousselme, B.; Tran, P. D.; Guillet, N.; Métayé, R.; Fihri, A.; Palacin, S.; Fontecave, M. From hydrogenases to noble metal-free catalytic nanomaterials for H2 production and uptake. Science 2009, 326, 1384–1387.
Turner, J. A. Sustainable hydrogen production. Science 2004, 305, 972–974.
Warren, S. C.; Voïtchovsky, K.; Dotan, H.; Leroy, C. M.; Cornuz, M.; Stellacci, F.; Hébert, C.; Rothschild, A.; Grätzel, M. Identifying champion nanostructures for solar water-splitting. Nat. Mater. 2013, 12, 842–849.
Karunadasa, H. I.; Chang, C. J.; Long, J. R. A molecular molybdenum-oxo catalyst for generating hydrogen from water. Nature 2010, 464, 1329–1333.
Xu, Y. T.; Xiao, X. F.; Ye, Z. M.; Zhao, S. L.; Shen, R. A.; He, C. T.; Zhang, J. P.; Li, Y. D.; Chen, X. M. Cage-confinement pyrolysis route to ultrasmall tungsten carbide nanoparticles for efficient electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 2017, 139, 5285–5288.
Wang, J.; Liu, D. F.; Qi, X. Q.; Xiong, K.; Li, L.; Wei, Z. D. Insight into the effect of CaMnO3 support on the catalytic performance of platinum catalysts. Chem. Eng. Sci. 2015, 135, 179–186.
Yin, H. J.; Zhao, S. L.; Zhao, K.; Muqsit, A.; Tang, H. J.; Chang, L.; Zhao, H. J.; Gao, Y.; Tang, Z. Y. Ultrathin platinum nanowires grown on single-layered nickel hydroxide with high hydrogen evolution activity. Nat. Commun. 2015, 6, 6430.
Song, J. G.; Ryu, G. H.; Lee, S. J.; Sim, S.; Lee, C. W.; Choi, T.; Jung, H.; Kim, Y.; Lee, Z.; Myoung, J. M. et al. Controllable synthesis of molybdenum tungsten disulfide alloy for vertically composition-controlled multilayer. Nat. Commun. 2015, 6, 7817.
Morales-Guio, C. G.; Tilley, S. D.; Vrubel, H.; Grätzel, M.; Hu, X. L. Hydrogen evolution from a copper(I) oxide photocathode coated with an amorphous molybdenum sulphide catalyst. Nat. Commun. 2014, 5, 3059.
Kibsgaard, J.; Chen, Z. B.; Reinecke, B. N.; Jaramillo, T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969.
Chhowalla, M.; Shin, H. S.; Eda, G.; Li, L. J.; Loh, K. P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275.
Cheng, N.; Norouzi Banis, M.; Liu, J.; Riese, A.; Mu, S. C.; Li, R. Y.; Sham, T.-K.; Sun, X. L. Atomic scale enhancement of metal-support interactions between Pt and ZrC for highly stable electrocatalysts. Energy Environ. Sci. 2015, 8, 1450–1455.
Zhou, X. L.; Liu, Y.; Ju, H. X.; Pan, B. C.; Zhu, J. F.; Ding, T.; Wang, C. D.; Yang, Q. Design and epitaxial growth of MoSe2–NiSe vertical heteronanostructures with electronic modulation for enhanced hydrogen evolution reaction. Chem. Mater. 2016, 28, 1838–1846.
Wang, H. T.; Tsai, C.; Kong, D. S.; Chan, K.; Abild-Pedersen, F.; Nørskov, J. K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575.
Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2016, 26, 4661–4672.
Choi, C. L.; Feng, J.; Li, Y. G.; Wu, J.; Zak, A.; Tenne, R.; Dai, H. J. WS2 nanoflakes from nanotubes for electrocatalysis. Nano Res. 2013, 6, 921–928.
Liu, B.; Zhao, Y.-F.; Peng, H.-Q.; Zhang, Z.-Y.; Sit, C.-K.; Yuen, M.-F.; Zhang, T.-R.; Lee, C.-S.; Zhang, W.-J. Nickel–cobalt diselenide 3D mesoporous nanosheet networks supported on Ni foam: An all-pH highly efficient integrated electrocatalyst for hydrogen evolution. Adv. Mater. 2017, 29, 1606521.
Ma, L. B.; Hu, Y.; Chen, R. P.; Zhu, G. Y.; Chen, T.; Lv, H. L.; Wang, Y. R.; Liang, J.; Liu, H. X.; Yan, C. Z. et al. Self-assembled ultrathin NiCo2S4 nanoflakes grown on Ni foam as high-performance flexible electrodes for hydrogen evolution reaction in alkaline solution. Nano Energy 2016, 24, 139–147.
Zhang, X.; Zhang, Y.; Yu, B.-B.; Yin, X.-L.; Jiang, W.-J.; Jiang, Y.; Hu, J.-S.; Wan, L.-J. Physical vapor deposition of amorphous MoS2 nanosheet arrays on carbon cloth for highly reproducible large-area electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 19277–19281.
Chen, Y.-Y.; Zhang, Y.; Jiang, W.-J.; Zhang, X.; Dai, Z. H.; Wan, L.-J.; Hu, J.-S. Pomegranate-like N,P-doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution. ACS Nano 2016, 10, 8851–8860.
Kuang, M.; Han, P.; Wang, Q. H.; Li, J.; Zheng, G. F. CuCo hybrid oxides as bifunctional electrocatalyst for efficient water splitting. Adv. Funct. Mater. 2016, 26, 8555–8561.
Li, Y. H.; Liu, P. F.; Pan, L. F.; Wang, H. F.; Yang, Z. Z.; Zheng, L. R.; Hu, P.; Zhao, H. J.; Gu, L.; Yang, H. G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Commun. 2015, 6, 8064.
Yan, X. D.; Tian, L. H.; He, M.; Chen, X. B. Threedimensional crystalline/amorphous Co/Co3O4 core/shell nanosheets as efficient electrocatalysts for the hydrogen evolution reaction. Nano Lett. 2015, 15, 6015–6021.
Xu, Y.-F.; Gao, M.-R.; Zheng, Y.-R.; Jiang, J.; Yu, S.-H. Nickel/nickel(II) oxide nanoparticles anchored onto cobalt(IV) diselenide nanobelts for the electrochemical production of hydrogen. Angew. Chem., Int. Ed. 2013, 52, 8546–8550.
Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J. G.; Guan, M. Y.; Lin, M.-C.; Zhang, B.; Hu, Y. F.; Wang, D.-Y.; Yang, J. et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695.
Suib, S. L. Porous manganese oxide octahedral molecular sieves and octahedral layered materials. Acc. Chem. Res. 2008, 41, 479–487.
Xu, G.-L.; Xu, Y.-F.; Fang, J.-C.; Fu, F.; Sun, H.; Huang, L.; Yang, S. H.; Sun, S.-G. Facile synthesis of hierarchical micro/nanostructured MnO material and its excellent lithium storage property and high performance as anode in a MnO/ LiNi0.5Mn1.5O4–δ lithium ion battery. ACS Appl. Mater. Interfaces 2013, 5, 6316–6323.
Zhang, K. J.; Han, P. X.; Gu, L.; Zhang, L. X.; Liu, Z. H.; Kong, Q. S.; Zhang, C. J.; Dong, S. M.; Zhang, Z. Y.; Yao, J. H. et al. Synthesis of nitrogen-doped MnO/graphene nanosheets hybrid material for lithium ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 658–664.
Reddy, A. L. M.; Shaijumon, M. M.; Gowda, S. R.; Ajayan, P. M. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett. 2009, 9, 1002–1006.
Liang, S. H.; Teng, F.; Bulgan, G.; Zong, R. L.; Zhu, Y. F. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C 2008, 112, 5307–5315.
Pinaud, B. A.; Chen, Z. B.; Abram, D. N.; Jaramillo, T. F. Thin films of sodium birnessite-type MnO2: Optical properties, electronic band structure, and solar photoelectrochemistry. J. Phys. Chem. C 2011, 115, 11830–11838.
Robinson, D. M.; Go, Y. B.; Mui, M.; Gardner, G.; Zhang, Z. J.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G. C. Photochemical water oxidation by crystalline polymorphs of manganese oxides: Structural requirements for catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501.
Takashima, T.; Hashimoto, K.; Nakamura, R. Inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J. Am. Chem. Soc. 2012, 134, 18153–18156.
Indra, A.; Menezes, P. W.; Zaharieva, I.; Baktash, E.; Pfrommer, J.; Schwarze, M.; Dau, H.; Driess, M. Active mixed-valent MnOx water oxidation catalysts through partial oxidation (corrosion) of nanostructured MnO particles. Angew. Chem., Int. Ed. 2013, 52, 13206–13210.
Zhou, F. L.; Izgorodin, A.; Hocking, R. K.; Spiccia, L.; MacFarlane, D. R. Electrodeposited MnOx films from ionic liquid for electrocatalytic water oxidation. Adv. Energy Mater. 2012, 2, 1013–1021.
Su, H. Y.; Gorlin, Y.; Man, I. C.; Calle-Vallejo, F.; Nørskov, J. K.; Jaramillo, T. F.; Rossmeisl, J. Identifying active surface phases for metal oxide electrocatalysts: a study of manganese oxide bi-functional catalysts for oxygen reduction and water oxidation catalysis. Phys. Chem. Chem. Phys. 2012, 14, 14010–14022.
El-Sawy, A. M.; King’ondu, C. K.; Kuo, C. H.; Kriz, D. A.; Guild, C. J.; Meng, Y. T.; Frueh, S. J.; Dharmarathna, S.; Ehrlich, S. N.; Suib, S. L. X-ray absorption spectroscopic study of a highly thermally stable manganese oxide octahedral molecular sieve (OMS-2) with high oxygen reduction reaction activity. Chem. Mater. 2014, 26, 5752–5760.
Xiao, W.; Wang, D. L.; Lou, X. W. Shape-controlled synthesis of MnO2 nanostructures with enhanced electrocatalytic activity for oxygen reduction. J. Phys. Chem. C 2010, 114, 1694–1700.
Xiao, Y. P.; Jiang, W. J.; Wan, S.; Zhang, X.; Hu, J. S.; Wei, Z. D.; Wan, L. J. Self-deposition of Pt nanocrystals on Mn3O4 coated carbon nanotubes for enhanced oxygen reduction electrocatalysis. J. Mater. Chem. A 2013, 1, 7463–7468.
Li, L.; Feng, X. H.; Nie, Y.; Chen, S. G.; Shi, F.; Xiong, K.; Ding, W.; Qi, X. Q.; Hu, J. S.; Wei, Z. D. et al. Insight into the effect of oxygen vacancy concentration on the catalytic performance of MnO2. ACS Catal. 2015, 5, 4825–4832.
Meng, Y. T.; Song, W. Q.; Huang, H.; Ren, Z.; Chen, S. Y.; Suib, S. L. Structure–property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electro-chemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464.
Gorlin, Y.; Lassalle-Kaiser, B.; Benck, J. D.; Gul, S.; Webb, S. M.; Yachandra, V. K.; Yano, J.; Jaramillo, T. F. In situ X-ray absorption spectroscopy investigation of a bifunctional manganese oxide catalyst with high activity for electrochemical water oxidation and oxygen reduction. J. Am. Chem. Soc. 2013, 135, 8525–8534.
Ray, C.; Dutta, S.; Negishi, Y.; Pal, T. A new stable Pd-Mn3O4 nanocomposite as an efficient electrocatalyst for the hydrogen evolution reaction. Chem. Commun. 2016, 52, 6095–6098.
Cheng, F. Y.; Su, Y.; Liang, J.; Tao, Z. L.; Chen, J. MnO2- based nanostructures as catalysts for electrochemical oxygen reduction in alkaline media. Chem. Mater. 2010, 22, 898–905.
Hou, Y.; Cheng, Y. W.; Hobson, T.; Liu, J. Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/ conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett. 2010, 10, 2727–2733.
Chen, P.-C.; Shen, G. Z.; Shi, Y.; Chen, H. T.; Zhou, C. W. Preparation and characterization of flexible asymmetric supercapacitors based on transition-metal-oxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes. ACS Nano 2010, 4, 4403–4411.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Nørskov, J. K.; Bligaard, T.; Logadottir, A.; Kitchin, J. R.; Chen, J. G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26.
Zhang, W.; Wen, X.; Yang, S.; Berta, Y.; Wang, Z. L. Single-crystalline scroll-type nanotube arrays of copper hydroxide synthesized at room temperature. Adv. Mater. 2003, 15, 822–825.
Jin, X. B.; Zhou, W. Z.; Zhang, S. W.; Chen, G. Z. Nanoscale microelectrochemical cells on carbon nanotubes. Small 2007, 3, 1513–1517.
Yan, J.; Fan, Z. J.; Wei, T.; Qian, W. Z.; Zhang, M. L.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833.
Zhao, X. D.; Fan, H. M.; Luo, J.; Ding, J.; Liu, X. Y.; Zou, B. S.; Feng, Y. P. Electrically adjustable, super adhesive force of a superhydrophobic aligned MnO2 nanotube membrane. Adv. Funct. Mater. 2011, 21, 184–190.
Julien, C.; Massot, M.; Baddour-Hadjean, R.; Franger, S.; Bach, S.; Pereira-Ramos, J. P. Raman spectra of birnessite manganese dioxides. Solid State Ionics 2003, 159, 345–356.
Cheng, S.; Yang, L. F.; Chen, D. C.; Ji, X.; Jiang, Z. J.; Ding, D.; Liu, M. L. Phase evolution of an alpha MnO2-based electrode for pseudo-capacitors probed by in operando Raman spectroscopy. Nano Energy 2014, 9, 161–167.
Audi, A. A.; Sherwood, P. M. A. Valence-band X-ray photoelectron spectroscopic studies of manganese and its oxides interpreted by cluster and band structure calculations. Surf. Interface Anal. 2002, 33, 274–282.
Huang, H. W.; Yu, Q.; Peng, X. S.; Ye, Z. Z. Single-unitcell thick Mn3O4 nanosheets. Chem. Commun. 2011, 47, 12831–12833.
Portehault, D.; Cassaignon, S.; Baudrin, E.; Jolivet, J. P. Structural and morphological control of manganese oxide nanoparticles upon soft aqueous precipitation through MnO4 –/Mn2+ reaction. J. Mater. Chem. 2009, 19, 2407–2416.
Oku, M.; Hirokawa, K.; Ikeda, S. X-ray photoelectron spectroscopy of manganese–oxygen systems. J. Electron Spectrosc. Relat. Phenom. 1975, 7, 465–473.
Chigane, M.; Ishikawa, M. Manganese oxide thin film preparation by potentiostatic electrolyses and electrochromism. J. Electrochem. Soc. 2000, 147, 2246–2251.
Brown, K. A.; He, S.; Eichelsdoerfer, D. J.; Huang, M. C.; Levy, I.; Lee, H.; Ryu, S.; Irvin, P.; Mendez-Arroyo, J.; Eom, C.-B. et al. Giant conductivity switching of LaAlO3/SrTiO3 heterointerfaces governed by surface protonation. Nat. Commun. 2016, 7, 10681–10686.
Cheng, J.; Lu, Y.; Qiu, K. W.; Yan, H. L.; Xu, J. Y.; Han, L.; Liu, X. M.; Luo, J. S.; Kim, J. K.; Luo, Y. S. Hierarchical core/shell NiCo2O4@NiCo2O4 nanocactus arrays with dualfunctionalities for highperformance supercapacitors and Li-ion batteries. Sci. Rep. 2015, 5, 12099.
Hinnemann, B.; Moses, P. G.; Bonde, J.; Jørgensen, K. P.; Nielsen, J. H.; Horch, S.; Chorkendorff, I.; Nørskov, J. K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309.
Sheng, W. C.; Myint, M.; Chen, J. G.; Yan, Y. S. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6, 1509–1512.
Acknowledgements
This work was financially supported by the National Basic Research Program of China (No. 2015CB932302), the National Key Research and Development Program of China (No. 2016YFB0101200), the National Natural Science Foundation of China (Nos. 91645123, 21573249, 21703257 and 21773263), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB12020100). We thank Dr. Z. J. Zhao and Prof. F. Liu at the Center for Analysis and Testing, ICCAS for their help for the XPS analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chen, L., Zhang, X., Jiang, W. et al. In situ transformation of Cu2O@MnO2 to Cu@Mn(OH)2 nanosheet-on-nanowire arrays for efficient hydrogen evolution. Nano Res. 11, 1798–1809 (2018). https://doi.org/10.1007/s12274-017-1798-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1798-6