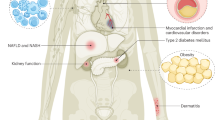
Abstract
Protein tyrosine phosphatases (PTPs) have an emerging paradigm for the development of antidiabetic drugs. Herein, we provide a comprehensive overview of the relevance of PTPs to type 2 diabetes (T2D) and the therapeutic opportunities thereof, while critically evaluating the potential challenges for PTP inhibitors to be next generation antidiabetics. This review briefly discusses the structure and function of PTPs. An account of importance and relevance of PTPs in various human diseases is presented with special attention to diabetes. The PTPs relevant to T2D have been targeted by small molecule inhibitors such as natural products and synthetic compounds as well as antisense nucleic acids. This review will give better understanding of the important concepts helpful in outlining the strategies for the development of new therapeutic agents with promising antidiabetic activities.






Similar content being viewed by others
References
Alonso A, Pulido R (2016) The extended human PTPome: a growing tyrosine phosphatase family. FEBS J 283:1404–1429. https://doi.org/10.1111/febs.13600
Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T (2004) Protein tyrosine phosphatases in the human genome. Cell 117:699–711. https://doi.org/10.1016/j.cell.2004.05.018
Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Møller NP (2001) Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol 21:7117–7136. https://doi.org/10.1128/MCB.21.21.7117-7136.2001
Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, Davies J, Vollmer S (2018) Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 41:963–970. https://doi.org/10.2337/dc17-1962
Brenachot X, Ramadori G, Ioris RM, Veyrat-Durebex C, Altirriba J, Aras E, Ljubicic S, Kohno D, Fabbiano S, Clement S, Goossens N, Trajkovski M, Harroch S, Negro F, Coppari R (2017) Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat Comm 8:1820. https://doi.org/10.1038/s41467-017-02074-2
Chagnon MJ, Elchebly M, Uetani N, Dombrowski L, Cheng A, Mooney RA, Marette A, Tremblay ML (2006) Altered glucose homeostasis in mice lacking the receptor protein tyrosine phosphatase sigma. Can J Physiol Pharmacol 84:755–763. https://doi.org/10.1139/y06-020
Cho CY, Koo SH, Wang Y, Callaway S, Hedrick S, Mak PA, Orth AP, Peters EC, Saez E, Montminy M, Chanda SPG, SK, (2006) Identification of the tyrosine phosphatase PTP-MEG2 as an antagonist of hepatic insulin signaling. Cell Metab 3:367–378. https://doi.org/10.1016/j.cmet.2006.03.006
Clampit JE, Meuth JL, Smith HT, Reilly RM, Jirousek MR, Trevillyan JM, Rondinone CM (2003) Reduction of protein-tyrosine phosphatase-1B increases insulin signaling in FAO hepatoma cells. Biochem Biophys Res Comm 300:261–267. https://doi.org/10.1016/S0006-291X(02)02839-5
Colberg SR, Grieco CR (2009) Exercise in the treatment and prevention of diabetes. Cur Sports Med Rep 8:169–175. https://doi.org/10.1249/JSR.0b013e3181ae0654
Crunkhorn S (2017) Protein tyrosine phosphatase inhibitor reverses diabetes. Nat Rev Drug Discov 16:312–313. https://doi.org/10.1038/nrd.2017.73
Delibegovic M, Bence KK, Mody N, Hong EG, Ko HJ, Kim JK, Kahn BB, Neel BG (2007) Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol 27:7727–7734. https://doi.org/10.1128/MCB.00959-07
Desouza SR, Olson MC, Tinucci SL, Sinner EK, Flynn RS, Marshall QF, Jakubowski HV, Mcintee EJ (2020) SAR of non-hydrolysable analogs of pyridoxal 5′-phosphate against low molecular weight protein tyrosine phosphatase isoforms. Bioorg Med Chem Lett 30:127342. https://doi.org/10.1016/j.bmcl.2020.127342
Digenio A, Pham NC, Watts LM, Morgan ES, Jung SW, Baker BF, Geary RS, Bhanot S (2018) Antisense inhibition of protein tyrosine phosphatase 1B with IONIS-PTP-1B Rx improves insulin sensitivity and reduces weight in overweight patients with type 2 diabetes. Diabetes Care 41:807–814. https://doi.org/10.2337/dc17-2132
Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548. https://doi.org/10.1126/science.283.5407.1544
Eleftheriou P, Geronikaki A, Petrou A (2019) PTP1b inhibition, a promising approach for the treatment of diabetes type II. Curr Topics Med Chem 19:246–263. https://doi.org/10.2174/1568026619666190201152153
Emanuelli B, Eberlé D, Suzuki R, Kahn CR (2008) Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc Natl Acad Sci USA 105:3545–3550. https://doi.org/10.1073/pnas.0712275105
Figueiredo A, Leal EC, Carvalho E (2020) Protein tyrosine phosphatase 1B inhibition as a potential therapeutic target for chronic wounds in diabetes. Pharmacol Res 159:104977. https://doi.org/10.1016/j.phrs.2020.104977
Fukushima A, Loh K, Galic S, Fam B, Shields B, Wiede F, Tremblay ML, Watt MJ, Andrikopoulos S, Tiganis T (2010) T-Cell protein tyrosine phosphatase attenuates STAT3 and insulin signaling in the liver to regulate gluconeogenesis. Diabetes 59:1906–1914. https://doi.org/10.2337/db09-1365
Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, Tiganis T (2005) Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol 25:819–829. https://doi.org/10.1128/MCB.25.2.819-829.2005
He R, Wang J, Yu ZH, Zhang RY, Liu S, Wu L, Zhang ZY (2016) Inhibition of low molecular weight protein tyrosine phosphatase by an induced-fit mechanism. J Med Chem 59:9094–9106. https://doi.org/10.1021/acs.jmedchem.6b00993
He RJ, Yu ZH, Zhang RY, Zhang ZY (2014) Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin 35:1227–1246. https://doi.org/10.1038/aps.2014.80
Hendriks WJAJ, Pulido R (2013) Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochim Biophys Acta 1832:1673–1696. https://doi.org/10.1016/j.bbadis.2013.05.022
Hendriks W, Bourgonje A, Leenders W, Pulido R (2018) Proteinaceous regulators and inhibitors of protein tyrosine phosphatases. Molecules 23:395. https://doi.org/10.3390/molecules23020395
Huang DW, Sherman BT, Lempicki RA (2009a) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. https://doi.org/10.1093/nar/gkn923
Huang DW, Sherman BT, Lempicki RA (2009b) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Hussain H, Green IR, Abbas G, Adekenov SM, Hussain W, Ali I (2019a) Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: patent review (2015–2018). Expert Opin Ther Pat 29:689–702. https://doi.org/10.1080/13543776.2019.1655542
Hussain RM, Neiweem AE, Kansara V, Harris A, Ciulla TA (2019b) Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert opin investig drugs 28:861–869. https://doi.org/10.1080/13543784.2019.1667333
Kaminski A, Welters HJ, Kaminski ER, Morgan NG (2009) Human and rodent pancreatic β-cells express IL-4 receptors and IL-4 protects against β-cell apoptosis by activation of the PI3K and JAK/STAT pathways. Biosci Rep 30:169–175. https://doi.org/10.1042/BSR20090021
Kharroubi AT, Darwish HM (2015) Diabetes mellitus: the epidemic of the century. World J Diabetes 6:850–867. https://doi.org/10.4239/wjd.v6.i6.850
Khursheed R, Singh SK, Wadhwa S, Kapoor B, Gulati M, Kumar R, Ramanunny AK, Awasthi A, Dua K (2019) Treatment strategies against diabetes: success so far and challenges ahead. Eur J Pharmacol 862:172625. https://doi.org/10.1016/j.ejphar.2019.172625
Kruger J, Brachs S, Trappiel M, Kintscher U, Meyborg H, Wellnhofer E, Thone-Reineke C, Stawowy P, Ostman A, Birkenfeld AL, Bohmer FD, Kappert K (2015) Enhanced insulin signaling in density-enhanced phosphatase-1 (DEP-1) knockout mice. Mol Metab 4:325–336. https://doi.org/10.1016/j.molmet.2015.02.001
Krüger J, Wellnhofer E, Meyborg H, Stawowy P, Östman A, Kintscher U, Kappert K (2016) Inhibition of Src homology 2 domain-containing phosphatase 1 increases insulin sensitivity in high-fat diet-induced insulin-resistant mice. FEBS Open Bio 6:179–189. https://doi.org/10.1002/2211-5463.12000
Kulas DT, Zhang WR, Goldstein BJ, Furlanetto RW, Mooney RA (1995) Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J Biol Chem 270:2435–2438. https://doi.org/10.1074/jbc.270.6.2435
Li J, Sipple J, Maynard S, Mehta PA, Rose SR, Davies SM, Pang Q (2012) Fanconi anemia links reactive oxygen species to insulin resistance and obesity. Antioxid Redox Signal 17:1083–1098. https://doi.org/10.1089/ars.2011.4417
Li Y, Duan B, Li Y, Yu S, Wang Y (2020) The isoflavonoid calycosin inhibits inflammation and enhances beta cell function in gestational diabetes mellitus by suppressing RNF38 expression. Immunopharmacol Immunotoxicol 42:366–372. https://doi.org/10.1080/08923973.2020.1782426
Liang XH, Sun H, Shen W, Wang S, Yao J, Migawa MT, Bui HH, Damle SS, Riney S, Graham MJ, Crooke RM, Crooke ST (2017) Antisense oligonucleotides targeting translation inhibitory elements in 5’ UTRs can selectively increase protein levels. Nucleic Acids Res 45:9528–9546. https://doi.org/10.1093/nar/gkx632
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF (2020) Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 10:14790. https://doi.org/10.1038/s41598-020-71908-9
Matsuo K, Delibegovic M, Matsuo I, Nagata N, Liu S, Bettaieb A, Xi Y, Araki K, Yang W, Kahn BB, Neel BG, Haj FG (2010) Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. J Biol Chem 285:39750–39758. https://doi.org/10.1074/jbc.M110.153734
Mccullough BS, Batsomboon P, Hutchinson KB, Dudley GB, Barrios AM (2019) Synthesis and PTP inhibitory activity of illudalic acid and its methyl ether, with insights into selectivity for LAR PTP over other tyrosine phosphatases under physiologically relevant conditions. J Nat Prod 82:3386–3393. https://doi.org/10.1021/acs.jnatprod.9b00663
Meng TC, Lou YW, Chen Y, Hsu S, Huang YF (2006) Cys-oxidation of protein tyrosine phosphatases: its role in regulation of signal transduction and its involvement in human cancers. J Cancer Mol 2:9–16. https://doi.org/10.29685/JCM.200602.0001
Monteiro LF, Ferruzo PYM, Russo LC, Farias JO, Forti FL (2019) DUSP3/VHR: a druggable dual phosphatase for human diseases. Rev Physiol Biochem Pharmacol 176:1–35. https://doi.org/10.1007/112_2018_12
Moore F, Colli ML, Cnop M, Esteve MI, Cardozo AK, Cunha DA, Bugliani M, Marchetti P, Eizirik DL (2009) PTPN2, a candidate gene for type 1 diabetes, modulates interferon-γ–induced pancreatic β-cell apoptosis. Diabetes 58:1283–1291. https://doi.org/10.2337/db08-1510
Paulsen CE, Carroll KS (2013) Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 113:4633–4679. https://doi.org/10.1021/cr300163e
Peti W, Page R (2015) Strategies to make protein serine/threonine (PP1, calcineurin) and tyrosine phosphatases (PTP1B) druggable: achieving specificity by targeting substrate and regulatory protein interaction sites. Bioorg Med Chem 23:2781–2785. https://doi.org/10.1016/j.bmc.2015.02.040
Seo H, Cho S (2015) PTP inhibitor V inhibits dual-specificity phosphatase 22 (DUSP22) activity. Bull Kor Chem Soc 36:2375–2378. https://doi.org/10.1002/bkcs.10444
Shi L, Zhang W, Zhou YY, Zhang YN, Li JY, Hu LH, Li J (2008) Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur J Pharmacol 584:21–29. https://doi.org/10.1016/j.ejphar.2008.01.020
Shintani T, Higashi S, Takeuchi Y, Gaudio E, Trapasso F, Fusco A, Noda M (2015) The R3 receptor-like protein tyrosine phosphatase subfamily inhibits insulin signalling by dephosphorylating the insulin receptor at specific sites. J Biochem 158:235–243. https://doi.org/10.1093/jb/mvv045
Stanford SM, Aleshin AE, Zhang V, Ardecky RJ, Hedrick MP, Zou J, Ganji SR, Bliss MR, Yamamoto F, Bobkov AA, Kiselar J, Liu Y, Cadwell GW, Khare S, Yu J, Barquilla A, Chung TDY, Mustelin T, Schenk S, Bankston LA, Liddington RC, Pinkerton AB, Bottini N (2017) Diabetes reversal by inhibition of the low-molecular-weight tyrosine phosphatase. Nat Chem Biol 13:624–632. https://doi.org/10.1038/nchembio.2344
Tautz L, Pellecchia M, Mustelin T (2006) Targeting the PTPome in human disease. Expert Opin Ther Targets 10:157–177. https://doi.org/10.1517/14728222.10.1.157
Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7:833–846. https://doi.org/10.1038/nrm2039
Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, Yang L, Musumeci L, Francis D, Landskron J, Tasken K, Tremblay ML, Lie BA, Page R, Mustelin T, Rahmouni S, Rickert RC, Tautz L (2012) LYP inhibits T-cell activation when dissociated from CSK. Nat Chem Biol 8:437–446. https://doi.org/10.1038/nchembio.916
Wang LJ, Jiang B, Wu N, Wang SY, Shi DY (2015) Natural and semisynthetic protein tyrosine phosphatase 1B (PTP1B) inhibitors as anti-diabetic agents. RSC Advances 5:48822–48834. https://doi.org/10.1039/C5RA01754H
Wang M, Li X, Dong L, Chen X, Xu W, Wang R (2016) Virtual screening, optimization, and identification of a novel specific PTP-MEG2 inhibitor with potential therapy for T2DM. Oncotarget 7:50828–50834. https://doi.org/10.18632/oncotarget.10341
Wu J, Sun Y, Zhou H, Ma Y, Wang R (2020) Design, synthesis, biological evaluation and molecular dynamics simulation studies of (R)-5-methylthiazolidin-4-one derivatives as megakaryocyte protein tyrosine phosphatase 2 (PTP-MEG2) inhibitors for the treatment of type 2 diabetes. J Biomol Struct Dyn 38:3156–3165. https://doi.org/10.1002/1873-3468.13537
Wu J, Tao W, Bu D, Zhao Y, Zhang T, Chong D, Xue B, Xing Z, Li C (2019) Egr-1 transcriptionally activates protein phosphatase PTP1B to facilitate hyperinsulinemia-induced insulin resistance in the liver in type 2 diabetes. FEBS Lett 593:3054–3063. https://doi.org/10.1002/1873-3468.13537
Yoon SY, Kang HJ, Ahn D, Hwang JY, Kwon SJ, Chung SJ (2019) Identification of chebulinic acid as a dual targeting inhibitor of protein tyrosine phosphatases relevant to insulin resistance. Bioorg Chem 90:103087. https://doi.org/10.1016/j.bioorg.2019.103087
Yoon SY, Lee JH, Kwon SJ, Kang HJ, Chung SJ (2018) Ginkgolic acid as a dual-targeting inhibitor for protein tyrosine phosphatases relevant to insulin resistance. Bioorg Chem 81:264–269. https://doi.org/10.1016/j.bioorg.2018.08.011
Zabolotny JM, Kim YB, Peroni OD, Kim JK, Pani MA, Boss O, Klaman LD, Kamatkar S, Shulman GI, Kahn BB, Neel BG (2001) Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc Natl Acad Sci USA 98:5187–5192. https://doi.org/10.1073/pnas.071050398
Zhang ZY (2003) Chemical and mechanistic approaches to the study of protein tyrosine phosphatases. Acc Chem Res 36:385–392. https://doi.org/10.1021/ar020122r
Zhang ZY, Dixon JE (1993) Active site labeling of the Yersinia protein tyrosine phosphatase: the determination of the pKa of the active site cysteine and the function of the conserved histidine 402. Biochem 32:9340–9345. https://doi.org/10.1021/bi00087a012
Zhang ZY, Dodd GT, Tiganis T (2015) Protein tyrosine phosphatases in hypothalamic insulin and Leptin signaling. Trends pharmacol sci 36:661–674. https://doi.org/10.1016/j.tips.2015.07.003
Zhao BT, Nguyen DH, Le DD, Choi JS, Min BS, Woo MH (2018) Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch Pharm Res 41:130–161. https://doi.org/10.1007/s12272-017-0997-8
Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF, Xie N, Wilcox D, Jacobson P, Frost L, Kroeger PE, Reilly RM, Koterski S, Opgenorth TJ, Ulrich RG, Crosby S, Butler M, Murray SF, Mckay RA, Bhanot S, Monia BP, Jirousek MR (2002) PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci USA 99:11357–11362. https://doi.org/10.1073/pnas.142298199
Acknowledgements
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (MSIT) (NRF2012M3A9C4048775, NRF-2017M3A9C8031995) and KRIBB Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, C., Kim, Y., Ahn, D. et al. Protein tyrosine phosphatases (PTPs) in diabetes: causes and therapeutic opportunities. Arch. Pharm. Res. 44, 310–321 (2021). https://doi.org/10.1007/s12272-021-01315-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-021-01315-9