Abstract
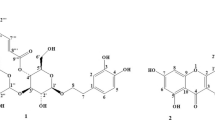
Three new neolignans, fordianoles A-C (1–3), characterized as (7S,8R)-4-hydroxy-3,3′,5′-trimethoxy-8′,9′-dinor-8,4′-oxyneolignan-7,7′,9-triol, (7R,8R)-4-hydroxy-3,3′,5′-trimethoxy-8′,9′-dinor-8,4′-oxyneolignan-7,7′,9-triol, and (7R,8R)-4-hydroxy-3,3′,5′-trimethoxy-8,4′-oxyneolignan-7,9,9′-triol-7′-one, together with an unusual γ-lactone, 3-(3,4-dihydroxyphenyl)-4-pentanolide (4), and twenty-five known compounds (5–29) were isolated from the aerial parts of Viburnum fordiae Hance. Their structures including absolute configurations were determined by spectroscopic and chemical methods. Among them, compounds 6, 7, 11–15, 17–28 were isolated from the Viburnum genus for the first time. The anti-inflammatory and antioxidant activities of all compounds were evaluated in vitro. Compounds 15, 19, 20 and 29 showed significant inhibitory activity on NO production in RAW264.7 cells stimulated by LPS with IC50 values ranging from 8.60 to 13.92 μM. Meanwhile, compounds 1–4, 15, 19, 20, 22, 23, 25, 26 and 29 exhibited varying antioxidant activities through DPPH, ABTS free radical scavenging and FRAP assays.


Similar content being viewed by others
References
Aasen AJ, Kimland B, Enzell CR (1973) Tobacco chemistry. 18. absolute configuration of (9R)-9-hydroxy-4,7E-megastigmadien-3-one (3-oxo-α-ionol). Acta Chem Scand 27:2107–2114
Arnoldi A, Merlini L (1985) Asymmetric synthesis of 3-methyl-2-phenyl-1,4-benzodioxanes. Absolute configuration of the neolignans eusiderin and eusiderin C and D. J Chem Soc, Perkin Trans 1:2555–2557
Benyahia S, Benayache S, Benayache F, León F, Quintana J, López M, Hernández JC, Estévez F, Bermejo J (2005) Cladocalol, a pentacyclic 28-nor-triterpene from Eucalyptus cladocalyx with cytotoxic activity. Phytochemistry 66:627–632
Besombes S, Robert D, Utille JP, Taravel FR, Mazeau K (2003) Molecular modeling of syringyl and p-hydroxyphenyl β-O-4 dimers. Comparative study of the computed and experimental conformational properties of lignin β-O-4 model compounds. J Agric Food Chem 51:34–42
Biswas SK (2016) Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid med cell longev 2016:5698931
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297:842–857
Carman RM, Robinson WT, Wallis CJ (2005) The 3-hydroxycineoles. Aust J Chem 58:785–791
Chen XQ, Shao LD, Pal M, Shen Y, Cheng X, Xu G, Peng LY, Wang K, Pan ZH, Li MM, Leng Y, He J, Zhao QS (2015) Hupehenols A-E, selective 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors from Viburnum hupehense. J Nat Prod 78:330–334
Deng XH, Zheng CJ, Wu Y, Qin LP (2013a) Chemical constituents of whole plant of Veronicastrum axillare (Sieb. et Zucc.) Yamazaki. Chin Pharm J 48:777–781
Deng Y, He JB, Guan KY, Zhu HJ (2013b) Studies on the chemical constituents of Cynanchum auriculatum. Nat Prod Res Dev 25:729–732
Gan ML, Zhang YL, Lin S, Liu MT, Song WX, Zi JC, Yang YC, Fan XN, Shi JG, Hu JF, Sun JD, Chen NH (2008) Glycosides from the root of Iodes cirrhosa. J Nat Prod 71:647–654
Gao L, Tian H, Lv PJ, Wang JP, Wang YF (2015) Chemical constituents of Sapium sebiferum leaves. Zhongguo Zhongyao Zazhi 40:1518–1522
He L, Yang SL, Wu DS, Cui T, Wei D, Ding ZT (2012) Coumarins from Skimmia arborescens and its anti-inflammatory effect. Zhongguo Zhongyao Zazhi 37:811–813
Huang Y, Shao HK, Li K, Liu SQ, Xie ST (2014) Chemical analysis and activity evaluation of anti-inflammatory constituents of Ficus microcarpa L. f. Chin Tradit Patent Med 36:1227–1233
Huang XX, Bai M, Zhou L, Lou LL, Liu QB, Zhang Y, Li LZ, Song SJ (2015) Food byproducts as a new and cheap source of bioactive compounds: lignans with antioxidant and anti-inflammatory properties from Crataegus pinnatifida seeds. J Agric Food Chem 63:7252–7260
In SJ, Seo KH, Song NY, Lee DS, Kim YC, Baek NI (2015) Lignans and neolignans from the stems of Vibrunum erosum and their neuroprotective and anti-inflammatory activity. Arch Pharm Res 38:26–34
Ishikawa T, Fujimatu E, Kitajima J (2002) Water-soluble constituents of anise: new glucosides of anethole glycol and its related compounds. Chem Pharm Bull 50:1460–1466
Iwagawa T, Yaguchi S, Hase T, Okubo T, Kim M (1992) Gomojosides, labdane diterpenoids from Viburnum suspensum. Phytochemistry 31:1311–1315
Iwai K, Kim MY, Onodera A, Matsue H (2006) α-Glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem 54:4588–4592
Jang DS, Han AR, Park G, Jhon GJ, Seo EK (2004) Flavonoids and aromatic compounds from the rhizomes of Zingiber zerumbet. Arch Pharm Res 27:386–389
Jing WG, Wang ZM, Zhao Y, Fu J, Zhao XL, Liu A (2014) Chemical constituents from seeds of Brassica campestris. Zhongguo Zhongyao Zazhi 39:2521–2525
Kikuzaki H, Hara S, Kawai Y, Nakatani N (1999) Antioxidative phenylpropanoids fromberries of Pimenta dioica. Phytochemistry 52:1307–1312
Li BZ, Wang BG, Jia ZJ (1998) Pentacyclic triterpenoids from Rubus xanthocarpus. Phytochemistry 49:2477–2481
Li H, Luo YG, Ma Y, Zeng J, Zhang X, Wang D, Hu CL (2013) Cytotoxic lignans from Viburnum foetidum. Arch Pharm Res 36:1211–1214
Li W, Tian XY, Xiao CJ, Jiang B (2014) Chemical constituents from the underground parts of Isodon phyllostachys (II). Chin Pharm J 49:1382–1385
Liu B, Liu MT, Gan ML, Zhao F, Wu XL, Yu Y, Yue ZG, Lin S, Wang SJ, Zhu CG, Shi JG (2012) Chemical constituents from roots of Machilus yaoshansis. Zhongguo Zhongyao Zazhi 37:1227–1231
Niu CS, Wang YD, Qu J, Yu SS, Li Y, Liu YB, Ma SG, Lv HN, Chen X, Xu S (2014) Chemical constituents from roots of Illicium majus. Zhongguo Zhongyao Zazhi 39:2689–2692
Ren J, Qin JJ, Cheng XR, Yan SK, Jin HZ, Zhang WD (2013) Five new sesquiterpene lactones from Inula hupehensis. Arch Pharm Res 36:1319–1325
Sadhu SK, Khatun A, Ohtsuki T, Ishibashi M (2008) Constituents from Hoya parasitica and their cell growth inhibitory activity. Planta Med 74:760–763
Shao ZX, Liu AQ, Lu B, Yao JZ (1992) Exploitation and utilization on Viburnum plants in Yunnan. Chin Wild Plant 11:29–31
Shen YC, Lin CL, Chien SC, Khalil AT, Ko CL, Wang CH (2004) Vibsane diterpenoids from the leaves and flowers of Viburnum odoratissimum. J Nat Prod 67:74–77
Tamura H, Fujita A, Takagi Y, Kitahara T, Mori K (1994) Simple synthesis of dehydrololiolide. Biosci Biotech Biochem 58:1902–1903
Wang JC, Li GZ, Lv N, Gao L, Cao L, Shen LG, Si JY (2016) Chemical constituents from the fruiting bodies of Cryptoporus volvatus. Arch Pharm Res 39:747–754
Wu HB, Zhao YN, Li DM, Lv XW, Wang WS (2013) Studies on the chemical constituents from Sambucus adnata Wall. Nat Prod Res Dev 25:345–348
Yilmaz BS, Altun ML, Orhan IE, Ergene B, Citoglu GS (2013) Enzyme inhibitory and antioxidant activities of Viburnum tinus L. relevant to its neuroprotective potential. Food Chem 141:582–588
Yin W, Song ZR, Liu JQ, Zhang GS (2015) Chemical constituents of Osmanthus fragrans. Zhongguo Zhongyao Zazhi 40:679–685
Zhang AL, Yu M, Xu HH, Si JP (2013a) Constituents of Dendrobium devonianum and their antioxidant activity. Zhongguo Zhongyao Zazhi 38:844–847
Zhang ZL, Zuo YM, Luo GM, Luo YJ, Wang YY, Yang YQ (2013b) Study on the chemical components of Gardenia jasminoides (II). Zhong Yao Cai 36:401–403
Zhao M, Chen LJ, Pei SC, Sun WJ, Li J, Zhang SJ (2014a) Chemical constituents of Ledum palustre (I). Chin Tradit Herbal Drugs 45:1532–1535
Zhao M, Wu S, Li J, Tang WX, Wang JL, Zhang SJ (2014b) Chemical constituents from Euphorbia lunulata. Zhongguo Zhongyao Zazhi 39:2289–2294
Zhonghuabencao Editorial Board (1999) Zhonghuabencao. Shanghai Scientific and Technical Press, Shanghai, pp 553–554
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31201563); Open foundation of green pesticides and biotechnology key laboratory of Guizhou University of Ministry of Education (2017GDGP0102/2017GDGP0103). We are also grateful to the Analytical Detective Center, Yangzhou University, for recording CD, IR, UV, MS and NMR spectra.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Shao, J., Zhao, C. et al. Chemical constituents from Viburnum fordiae Hance and their anti-inflammatory and antioxidant activities. Arch. Pharm. Res. 41, 625–632 (2018). https://doi.org/10.1007/s12272-018-1026-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1026-2