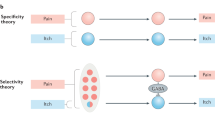
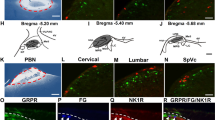
Abstract
Peripheral itch stimuli are transmitted by sensory neurons to the spinal cord dorsal horn, which then transmits the information to the brain. The molecular and cellular mechanisms within the dorsal horn for itch transmission have only been investigated and identified during the past ten years. This review covers the progress that has been made in identifying the peptide families in sensory neurons and the receptor families in dorsal horn neurons as putative itch transmitters, with a focus on gastrin-releasing peptide (GRP)—GRP receptor signaling. Also discussed are the signaling mechanisms, including opioids, by which various types of itch are transmitted and modulated, as well as the many conflicting results arising from recent studies.




Similar content being viewed by others
References
Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011, 26: 286–292.
Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007, 448: 700–703.
Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 2008, 60: 1–42.
Kroog GS, Jensen RT, Battey JF. Mammalian bombesin receptors. Med Res Rev 1995, 15: 389–417.
Decker MW, Towle AC, Bissette G, Mueller RA, Lauder JM, Nemeroff CB. Bombesin-like immunoreactivity in the central nervous system of capsaicin-treated rats: a radioimmunoassay and immunohistochemical study. Brain Res 1985, 342: 1–8.
Panula P, Hadjiconstantinou M, Yang HY, Costa E. Immunohistochemical localization of bombesin/gastrin-releasing peptide and substance P in primary sensory neurons. J Neurosci 1983, 3: 2021–2029.
Moody TW, Thoa NB, O’Donohue TL, Jacobowitz DM. Bombesin-like peptides in rat spinal cord: biochemical characterization, localization and mechanism of release. Life Sci 1981, 29: 2273–2279.
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 2009, 325: 1531–1534.
Lee H, Naughton NN, Woods JH, Ko MC. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol 2003, 14: 501–508.
Cowan A, Khunawat P, Zhu XZ, Gmerek DE. Effects of bombesin on behavior. Life Sci 1985, 37: 135–145.
Gmerek DE, Cowan A. Bombesin—a central mediator of pruritus? Br J Dermatol 1983, 109: 239.
Li MZ, Wang JS, Jiang DJ, Xiang CX, Wang FY, Zhang KH, et al. Molecular mapping of developing dorsal horn-enriched genes by microarray and dorsal/ventral subtractive screening. Dev Biol 2006, 292: 555–564.
Bromage PR, Camporesi EM, Durant PA, Nielsen CH. Nonrespiratory side effects of epidural morphine. Anesth Analg 1982, 61: 490–495.
Dahl JB, Jeppesen IS, Jorgensen H, Wetterslev J, Moiniche S. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology 1999, 91: 1919–1927.
Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 2011, 147: 447–458.
Abbadie C, Pan Y, Drake CT, Pasternak GW. Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 2000, 100: 141–153.
Moser HR, Giesler GJ, Jr. Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci 2013, 33: 6093–6101.
Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ, Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 2007, 27: 10007–10014.
Jansen NA, Giesler GJ, Jr. Response characteristics of pruriceptive and nociceptive trigeminoparabrachial tract neurons in the rat. J Neurophysiol 2015, 113: 58–70.
Moser HR, Giesler GJ, Jr. Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol 2014, 111: 1574–1589.
Millan MJ. Descending control of pain. Prog Neurobiol 2002, 66: 355–474.
Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984, 7: 309–338.
Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, et al. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci 2007, 27: 6045–6053.
Zhao ZQ, Liu XY, Jeffry J, Karunarathne WK, Li JL, Munanairi A, et al. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron 2014, 84: 821–834.
Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest 2013, 123: 4769–4780.
Barry DM, Li H, Liu XY, Shen KF, Liu XT, Wu ZY, et al. Critical evaluation of the expression of gastrin-releasing peptide in dorsal root ganglia and spinal cord. Mol Pain 2016, 12.
Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 2010, 68: 529–542.
Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med 2015, 21: 927–931.
Tirado-Sanchez A, Bonifaz A, Ponce-Olivera RM. Serum gastrin-releasing peptide levels correlate with disease severity and pruritus in patients with atopic dermatitis. Br J Dermatol 2015, 173: 298–300.
Kagami S, Sugaya M, Suga H, Morimura S, Kai H, Ohmatsu H, et al. Serum gastrin-releasing peptide levels correlate with pruritus in patients with atopic dermatitis. J Invest Dermatol 2013, 133: 1673–1675.
Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol 2017, 198: 2543–2555. .
Pereira PJ, Machado GD, Danesi GM, Canevese FF, Reddy VB, Pereira TC, et al. GRPR/PI3Kgamma: partners in central transmission of itch. J Neurosci 2015, 35: 16272–16281.
Sakamoto H, Matsuda K, Zuloaga DG, Hongu H, Wada E, Wada K, et al. Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat Neurosci 2008, 11: 634–636.
Hampton LL, Ladenheim EE, Akeson M, Way JM, Weber HC, Sutliff VE, et al. Loss of bombesin-induced feeding suppression in gastrin-releasing peptide receptor-deficient mice. Proc Natl Acad Sci U S A 1998, 95: 3188–3192.
Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci 2006, 7: 535–547.
Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci 2000, 12: 689–700.
Xu Y, Lopes C, Wende H, Guo Z, Cheng L, Birchmeier C, et al. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci 2013, 33: 14738–14748.
Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 2013, 78: 312–324.
Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014, 82: 573–586.
Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 2010, 65: 886–898.
Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014, 159: 1417–1432.
Phan NQ, Lotts T, Antal A, Bernhard JD, Stander S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm Venereol 2012, 92: 555–560.
Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 2010, 25: 1251–1257.
Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol 2005, 16: 3742–3747.
Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 2015, 85: 1289–1304.
Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 2015, 350: 550–554.
Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain 2012, 8: 52.
Zhao ZQ, Wan L, Liu XY, Huo FQ, Li H, Barry DM, et al. Cross-inhibition of NMBR and GRPR signaling maintains normal histaminergic itch transmission. J Neurosci 2014, 34: 12402–12414.
Mishra SK, Holzman S, Hoon MA. A nociceptive signaling role for neuromedin B. J Neurosci 2012, 32: 8686–8695.
Hong Y, Abbott FV. Behavioural effects of intraplantar injection of inflammatory mediators in the rat. Neuroscience 1994, 63: 827–836.
LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol 2011, 20: 778–782.
Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 2013, 340: 968–971.
Solorzano C, Villafuerte D, Meda K, Cevikbas F, Braz J, Sharif-Naeini R, et al. Primary afferent and spinal cord expression of gastrin-releasing Peptide: message, protein, and antibody concerns. J Neurosci 2015, 35: 648–657.
Wada E, Way J, Lebacq-Verheyden AM, Battey JF. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci 1990, 10: 2917–2930.
Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015, 18: 145–153.
Goswami SC, Thierry-Mieg D, Thierry-Mieg J, Mishra S, Hoon MA, Mannes AJ, et al. Itch-associated peptides: RNA-Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord. Mol Pain 2014, 10: 44.
Liu XY, Wan L, Huo FQ, Barry DM, Li H, Zhao ZQ, et al. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol Pain 2014, 10: 4.
Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest 2013, 123: 1513–1530.
Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest 2012, 122: 2195–2207.
Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A 2002, 99: 8360–8365.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445: 168–176.
Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 2013, 16: 174–182.
Bell AM, Gutierrez-Mecinas M, Polgar E, Todd AJ. Spinal neurons that contain gastrin-releasing peptide seldom express Fos or phosphorylate extracellular signal-regulated kinases in response to intradermal chloroquine. Mol Pain 2016, 12.
Cao X, Mercaldo V, Li P, Wu LJ, Zhuo M. Facilitation of the inhibitory transmission by gastrin-releasing peptide in the anterior cingulate cortex. Mol Pain 2010, 6: 52.
Kusube F, Tominaga M, Kawasaki H, Yamakura F, Naito H, Ogawa H, et al. Electrophysiological properties of brain-natriuretic peptide- and gastrin-releasing peptide-responsive dorsal horn neurons in spinal itch transmission. Neurosci Lett 2016, 627: 51–60.
Akiyama T, Carstens E. Neural processing of itch. Neuroscience 2013, 250: 697–714.
Rogoz K, Andersen HH, Lagerstrom MC, Kullander K. Multimodal use of calcitonin gene-related Peptide and substance p in itch and acute pain uncovered by the elimination of vesicular glutamate transporter 2 from transient receptor potential cation channel subfamily v member 1 neurons. J Neurosci 2014, 34: 14055–14068.
Acknowledgements
D.M.B. was supported by NIH-NIDA T32 Training Grant 5T32DA007261-23 and a W.M. Keck Fellowship. The research work was supported by NIH Grants 1R01AR056318-06, R21 NS088861-01A1, R01NS094344, R01 DA037261-01A1, and R56 AR064294-01A1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barry, D.M., Munanairi, A. & Chen, ZF. Spinal Mechanisms of Itch Transmission. Neurosci. Bull. 34, 156–164 (2018). https://doi.org/10.1007/s12264-017-0125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0125-2