Abstract
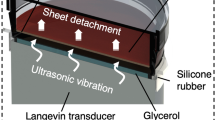
Bioengineering research and applications are supported by cell culture technologies that produce a large number of homogeneous cells. However, trypsin used in the general culture procedure for cell detachment decreases cell activity and culture efficiency. Furthermore, manually conducted culture procedures, especially pipetting after trypsin treatment, can induce inhomogeneous mechanical stress in cells, which may influence cellular functions. Alternate detachment methods using specialized culture devices without trypsin and/or manual pipetting have been reported. However, conventional trypsinization is still widely used. Diluted trypsin increases culture efficiency. Therefore, we developed a cell-detaching method using diluted trypsin and ultrasonic vibration for cell detachment from ubiquitous culture vessels. To demonstrate our concept, we used a T25 flask. Vibration of the culture surface was excited by ultrasonic waves propagated from an ultrasonic transducer placed under the flask. Using the proposed method, cells were completely detached by diluted trypsin, whereas 8.6% of cells remained on the flask with manual pipetting. The viability and proliferation of cells detached by the proposed method were higher than those of cells detached by the conventional method, owing to the low concentration of trypsin. Furthermore, glucose consumption after detachment showed no abnormality, eliminating possible oncogenesis. Two membrane proteins were quantified immediately after detachment and at 24 h of culture, and there were no differences between the detachment methods. Thus, we conclude that our proposed method improves culture efficiency without any adverse effects and ensures homogeneous mechanical stress on cells.
Similar content being viewed by others
References
Kino-Oka, M., N. Ogawa, R. Umegaki, and M. Taya (2005) Bioreactor design for successive culture of anchorage-dependent cells operated in an automated manner. Tissue Eng. 11: 535–545.
Kobayashi, T., K. Kan, K. Nishida, M. Yamato, and T. Okano (2013) Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials 34: 9010–9017.
Melero-Martin, J. M., M. A. Dowling, M. Smith, and M. Al-Rubeai (2006) Optimal in-vitro expansion of chondroprogenitor cells in monolayer culture. Biotechnol. Bioeng. 93: 519–533.
Punshon, G., D. S. Vara, K. M. Sales, and A. M. Seifalian, (2011) The long-term stability in gene expression of human endothelial cells permits the production of large numbers of cells suitable for use in regenerative medicine. Biotechnol. Appl. Biochem. 58: 371–375.
Jing, D., A. Parikh, J. M. Canty, and E. S. Tzanakakis (2008) Stem cells for heart cell therapies. Tissue Eng. Part B Rev. 14: 393–406.
Freshney, R. I., A. Capes-Davis, C. Gregory, and S. Przyborski (2011) Culture of animal cells: a manual of basic technique and specialized applications. Wiley Online Library.
Nakao, M., Y. Kurashina, C. Imashiro, and K. Takemura (2017) A method for collecting single cell suspensions using an ultrasonic pump. IEEE Trans. Biomed. Eng. 65: 224–231.
Umegaki, R., M. Kino-Oka, and M. Taya (2004) Assessment of cell detachment and growth potential of human keratinocyte based on observed changes in individual cell area during trypsinization. Biochem. Eng. J. 17: 49–55.
Kurashina, Y., Y. Hirano, C. Imashiro, K. Totani, J. Komotori, and K. Takemura (2017) Enzyme-free cell detachment mediated by resonance vibration with temperature modulation. Biotechnol. Bioeng. 114: 2279–2288.
Kurashina, Y., K. Takemura, J. Friend, S. Miyata, and J. Komotori (2016) Efficient subculture process for adherent cells by selective collection using cultivation substrate vibration. IEEE Trans. Biomed. Eng. 64: 580–587.
Mitomo, H., E. Asumi, S. Yasunobu, M. Yasutaka, N. Kenichi, K. Nakazawa, and K. Ijiro (2016) Fabrication of a novel cell culture system using DNA-grafted substrates and DNase. J. Biomed. Nanotechnol. 12: 286–295.
Batista, U., M. Garvas, M. Nemec, M. Schara, P. Veranič, and K. Tilen (2010) Effects of different detachment procedures on viability, nitroxide reduction kinetics and plasma membrane heterogeneity of V-79 cells. Cell Biol. Int. 34: 663–668.
Okano, T., N. Yamada, M. Okuhara, H. Sakai and Y. Sakurai (1995) Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 16: 297–303.
Papadopoulou, E. L., M. Barberoglou, V. Zorba, A. Manousaki, A. Pagkozidis, E. Stratakis, and C. Fotakis (2009) Reversible photoinduced wettability transition of hierarchical ZnO structures. J. Phys. Chem. C 113: 2891–2895.
Mittal, A., M. Pulina, S.Y. Hou, and S. Astrof (2013) Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev. Biol. 381: 73–82.
Lorenz, A., W. Just, M. Da, S. Cardoso, and G. Klotz (1988) Electroporation-mediated transfection of Acholeplasma laidlawii with mycoplasma virus Li and L3 DNA. J. Virol. 62: 3050–3052.
Hirai, H., R. Umegaki, M. Kino-Oka, and M. Taya (2002) Characterization of cellular motions through direct observation of individual cells at early stage in anchorage-dependent culture. J Biosci. Bioeng. 94: 351–356.
Kimura, Y., H. Okuda, and S. Ogita (1997) Effects of flavonoids isolated from scutellariae radix on fibrinolytic system induced by trypsin in human umbilical vein endothelial cells. J. Nat. Prod. 60: 598–601.
Huang, H. L., H. W. Hsing, T. C. Lai, Y. W. Chen, T. R. Lee, H. T. Chan, P. C. Lyu, C. L. Wu, Y. C. Lu, S. T. Lin, C. W. Lin, C. H. Lai, H. T. Chang, H. C. Chou, and H. L. Chan (2010) Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 17: 36.
Haeger, A., K. Wolf, M. M. Zegers, and P. Friedl (2015) Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 25: 556–566.
Nakajima, K., S. Honda, Y. Nakamura, F. López-Redondo, S. Kohsaka, M. Yamato, A. Kikuchi, and T. Okano (2001) Intact microglia are cultured and non-invasively harvested without pathological activation using a novel cultured cell recovery method. Biomaterials 22: 1213–1223.
Yang, M., N. Yang, S. Bi, X. He, L. Chen, Z. Zhu, Y. Gao, and Z. Du (2013) Micropatterned designs of thermoresponsive surfaces for modulating cell behaviors. Polym. Adv. Technol. 24: 1102–1109.
Kakegawa, T., N. Mochizuki, N. Sadr, H. Suzuki, and J. Fukuda (2013) Cell-Adhesive and cell-repulsive zwitterionic oligopeptides for micropatterning and rapid electrochemical detachment of cells. Tissue Eng. Part A. 19: 290–298.
Yoon, S. H. and M. R. K. Mofrad (2011) Cell adhesion and detachment on gold surfaces modified with a thiol-functionalized RGD peptide. Biomaterials 32: 7286–7296.
Imashiro, C., Y. Kurashina, T. Kuribara, M. Hirano, K. Totani, and K. Takemura, (2018) Cell patterning method on a clinically ubiquitous culture dish using acoustic pressure generated from resonance vibration of a disk-shaped ultrasonic transducer. IEEE Trans. Biomed. Eng. 111–118
Vanherberghen, B., O. Manneberg, A. Christakou, T. Frisk, M. Ohlin, H. M. Hertz, B. Önfelt, and M. Wiklund (2010) Ultrasound-controlled cell aggregation in a multi-well chip. Lab. Chip. 10: 2727–2732.
Wiklund M. (2012) Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators. Lab. Chip. 12: 2018–2028
Kino-Oka, M. and M. Taya (2009) Recent developments in processing systems for cell and tissue cultures toward therapeutic application. J. Biosci. Bioeng. 108: 267–276.
Burns, E. L., V. Suntzeff, and L. Loeb (1938) The development of sarcoma in mice injected with hormones or hormone-like substances. Am. J. Cancer 32: 534–544.
Cairns, R. A., I. S. Harris, and T. W. Mak (2011) Regulation of cancer cell metabolism. Nat. Rev. Cancer 11: 85–95.
Wang, C. Z., C. Z. Wang, G. J. Wang, M. L. Ho, Y. H. Wang, M. L. Yeh, and C. H. Chen (2010) Low-magnitude vertical vibration enhances myotube formation in C2C12 myoblasts. J. Appl. Physiol. 109: 840–848.
Hino, H., S. Hashimoto, Y. Takahashi, and H. Nakajima (2016) Effect of ultrasonic vibration on proliferation and differentiation of cells. Proceedings of The 20th World Multi-Conference on Systemics, Cybernetics and Informatics. July 5–8. Florida, USA.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP16H04259, JP17H07081, and 18J12482, and a Grant-in-Aid for JSPS Fellows. This work was also supported in part by a MEXT Grant-in-Aid for the Program for Leading Graduate Schools and JST Global Science Campus Program. It is acknowledged that Chikahiro Imashiro is a research fellow of the Japan Society for the Promotion of Science. Chikahiro Imashiro, Yuta Kurashina, and Kenjiro Takemura have a patent pending based on the work presented in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tauchi, H., Imashiro, C., Kuribara, T. et al. Effective and Intact Cell Detachment from a Clinically Ubiquitous Culture Flask by Combining Ultrasonic Wave Exposure and Diluted Trypsin. Biotechnol Bioproc E 24, 536–543 (2019). https://doi.org/10.1007/s12257-018-0491-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-018-0491-2