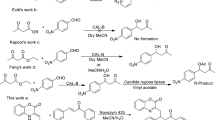
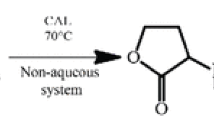
Abstract
Enzyme-catalyzed esterification of secondary alcohols and multi-hydroxyl compounds is one of the most valuable reactions in organic synthesis. However, it is often difficult to achieve high reaction rates and high regio-selectivities with commonly used enzymes such as lipases and proteases. One of the reasons may include bulky substituents of the secondary alcohols and multi-hydroxyl compounds (e.g., carbohydrates and flavonoids). The stereospecificity pocket of lipases, which is considered as a pocket for the binding of medium substituent, might not accept a large substituent due to steric hindrance. Thereby, this review has focused on the discussion about literature survey and structural feature of the most commonly used lipase (i.e., Candida antarctica lipase B (CAL-B)) and serine-protease (i.e., subtilisin) for acylation of secondary alcohols and complex molecules.
Similar content being viewed by others
References
Antonopoulou, I., S. Varriale, E. Topakas, U. Rova, P. Christakopoulos, and V. Faraco (2016) Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 100: 6519–6543.
de Araújo, M. E. M., Y. E. Franco, M. C. Messias, G. B. Longato, J. A. Pamphile, and P. d. O. Carvalho (2017) Biocatalytic synthesis of flavonoid esters by lipases and their biological benefits. Planta Med. 83: 7–22.
Gullón, B., T. A. Lú-Chau, M. T. Moreira, J. M. Lema, and G. Eibes (2017) Rutin: a review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol. 67: 220–235.
Scalbert, A., C. Manach, C. Morand, C. Rémésy, and L. Jiménez (2005) Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45: 287–306.
Bezbradica, D., M. Crovic, S. J Tanaskovic, N. Lukovic, M. Carevic, A. Milivojevic, and Z. Knezevic-Jugovic (2017) Enzymatic syntheses of esters-green chemistry for valuable food, fuel and fine chemicals. Curr. Org. Chem. 21: 104–138.
Cha, H.-J., E.-J. Seo, J.-W. Song, H.-J. Jo, A. R. Kumar, and J.-B. Park (2018) Simultaneous enzyme/whole-cell biotransformation of C18 ricinoleic acid into (R)-3-hydroxynonanoic acid, 9-hydroxynonanoic acid, and 1, 9-nonanedioic acid. Adv. Synth. Catal. 360: 696–703.
Jeon, E.-Y., J.-H. Seo, W.-R. Kang, M.-J. Kim, J.-H. Lee, D.-K. Oh, and J.-B. Park (2016) Simultaneous enzyme/wholecell biotransformation of plant oils into C9 carboxylic acids. ACS Catal. 6: 7547–7553.
Seo, E.-J., Y. J. Yeon, J.-H. Seo, J.-H. Lee, J. P. Boñgol, Y. Oh, J. M. Park, S.-M. Lim, C.-G. Lee, and J.-B. Park (2018) Enzyme/whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1, 9-nonanedioic acid. Bioresour. Technol. 251: 288–294.
Seo, J.-H., S.-W. Baek, J. Lee, and J.-B. Park (2017) Engineering Escherichia coli BL21 genome to improve the heptanoic acid tolerance by using CRISPR-Cas9 system. Biotechnol. Bioprocess Eng. 22: 231–238.
Yeon, Y. J. and J.-B. Park (2018) Regiospecific conversion of lipids and fatty acids through enzymatic cascade reactions. pp. 139–155. Lipid Modification by Enzymes and Engineered Microbes. Elsevier.
Bornscheuer, U. T. and R. J. Kazlauskas (2006) Hydrolases in organic synthesis: regio-and stereoselective biotransformations. John Wiley & Sons.
Carvalho, A. C. L. d. M., T. d. S. Fonseca, M. C. d. Mattos, M. d. C. F. d. Oliveira, T. L. G. d. Lemos, F. Molinari, D. Romano, and I. Serra (2015) Recent advances in lipase-mediated preparation of pharmaceuticals and their intermediates. Int. J. Mol. Sci. 16: 29682–29716.
Kim, J. (2017) Surface display of lipolytic enzyme, Lipase A and Lipase B of Bacillus subtilis on the Bacillus subtilis spore. Biotechnol. Bioprocess Eng. 22: 462–468.
Kirdi, R., N. B. Akacha, Y. Messaoudi, and M. Gargouri (2017) Enhanced synthesis of isoamyl acetate using liquid-gas biphasic system by the transesterification reaction of isoamyl alcohol obtained from fusel oil. Biotechnol. Bioprocess Eng. 22: 413–422.
Kourist, R., H. Brundiek, and U. T. Bornscheuer (2010) Protein engineering and discovery of lipases. Eur. J. Lipid. Sci. Technol. 112: 64–74.
Lee, H.-J., M. Haq, P. S. Saravana, Y.-N. Cho, and B.-S. Chun (2017) Omega-3 fatty acids concentrate production by enzymecatalyzed ethanolysis of supercritical CO2 extracted oyster oil. Biotechnol. Bioprocess Eng. 22: 518–528.
Schreck, S. D. and A. M. Grunden (2014) Biotechnological applications of halophilic lipases and thioesterases. Appl. Microbiol. Biotechnol. 98: 1011–1021.
Sheldon, R. A. and P. C. Pereira (2017) Biocatalysis engineering: the big picture. Chem. Soc. Rev. 46: 2678–2691.
Chen, H., X. Meng, X. Xu, W. Liu, and S. Li (2018) The molecular basis for lipase stereoselectivity. Appl. Microbiol. Biotechnol. 102: 3487–3495.
Kazlauskas, R. J. and A. N. Weissfloch (1997) A structure-based rationalization of the enantiopreference of subtilisin toward secondary alcohols and isosteric primary amines. J. Mol. Catal. B Enzym. 3: 65–72.
Schmid, R. D. and R. Verger (1998) Lipases: interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. Engl. 37: 1608–1633.
Kazlauskas, R. J., A. N. Weissfloch, A. T. Rappaport, and L. A. Cuccia (1991) A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 56: 2656–2665.
Orrenius, C., N. Öhrner, D. Rotticci, A. Mattson, K. Hult, and T. Norin (1995) Candida antarctica lipase B catalysed kinetic resolutions: Substrate structure requirements for the preparation of enantiomerically enriched secondary alcanols. Tetrahedron Asymmetry 6: 1217–1220.
Rotticci, D., F. Hæffner, C. Orrenius, T. Norin, and K. Hult (1998) Molecular recognition of sec-alcohol enantiomers by Candida antarctica lipase B. J. Mol. Catal. B Enzym. 5: 267–272.
Park, A., S. Kim, J. Park, S. Joe, B. Min, J. Oh, J. Song, S. Park, S. Park, and H. Lee (2016) Structural and experimental evidence for the enantiomeric recognition toward a bulky sec-alcohol by Candida antarctica lipase B. ACS Catal. 6: 7458–7465.
Woudenberg-van Oosterom, M., F. van Rantwijk, and R. A. Sheldon (1996) Regioselective acylation of disaccharides in tertbutyl alcohol catalyzed by Candida antarctica lipase. Biotechnol. Bioeng. 49: 328–333.
Rich, J. O., B. A. Bedell, and J. S. Dordick (1995) Controlling enzyme-catalyzed regioselectivity in sugar ester synthesis. Biotechnol. Bioeng. 45: 426–434.
Fersht, A. R., J.-P. Shi, J. Knill-Jones, D. M. Lowe, A. J. Wilkinson, D. M. Blow, P. Brick, P. Carter, M. M. Waye, and G. Winter (1985) Hydrogen bonding and biological specificity analysed by protein engineering. Nature 314: 235.
Magnusson, A. O., J. C. Rotticci-Mulder, A. Santagostino, and K. Hult (2005) Creating space for large secondary alcohols by rational redesign of Candida antarctica lipase B. ChemBioChem. 6: 1051–1056.
Magnusson, A. O., M. Takwa, A. Hamberg, and K. Hult (2005) An S-selective lipase was created by rational redesign and the enantioselectivity increased with temperature. Angew. Chem. Int. Ed. Engl. 44: 4582–4585.
Jeon, E.-Y., A.-H. Baek, U. T. Bornscheuer, and J.-B. Park (2015) Enzyme fusion for whole-cell biotransformation of long-chain sec-alcohols into esters. Appl. Microbiol. Biotechnol. 99: 6267–6275.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cha, HJ., Park, JB. & Park, S. Esterification of Secondary Alcohols and Multi-hydroxyl Compounds by Candida antarctica Lipase B and Subtilisin. Biotechnol Bioproc E 24, 41–47 (2019). https://doi.org/10.1007/s12257-018-0379-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-018-0379-1