Abstract
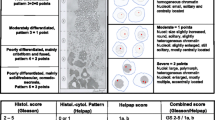
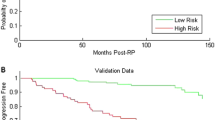
To test the agreement between high-grade PCa at RP and TMA, and the ability of TMA to predict BCR. Validation of concordance between tissue microarray (TMA) and radical prostatectomy (RP) high-grade prostate cancer (PCa) is crucial because latter determines the treated natural history of PCa. We hypothesized that TMA Gleason score is in agreement with RP pathology and capable of accurately predicting biochemical recurrence (BCR). Data were provided from a multi-institutional Canadian sample of 1333 TMA and RP specimens with complete clinicopathological data. First, rate of agreement between TMA and high-grade Gleason at RP or biopsy and RP was tested. Second, ability of RP, TMA and biopsy to predict BCR was compared. Multivariable (MVA) Cox regression models were fitted and BCR rates were illustrated with Kaplan-Meier plots. Agreement between RP and TMA and between RP and biopsy was 72.6% (95% CI:69.7–75.5) and 60.4% (95% CI:57.2–63.6), respectively. In MVA predicting BCR, the accuracy for RP, TMA and biopsy was 0.73, 0.72 and 0.68, respectively. TMA added discriminatory ability among exclusively low-grade Gleason RP patients (p = 0.02), but did not improve BCR discrimination in exclusive high-grade PCa RP patients (p = 0.8). TMA Gleason grade accurately reflects presence of high-grade Gleason in RP specimen, accurately predicts BCR rates after RP and improves prediction of BCR in low-grade Gleason patients at RP.



Similar content being viewed by others
References
Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Epstein JI, Cascinu S, Briganti A, Catto JW, Montorsi F, Montironi R (2016) Current Histopathologic and molecular Characterisations of prostate Cancer: towards individualised prognosis and therapies. European Urology 69(2):186–190. https://doi.org/10.1016/j.eururo.2015.05.041
Wittschieber D, Kollermann J, Schlomm T, Sauter G, Erbersdobler A (2010) Nuclear grading versus Gleason grading in small samples containing prostate cancer: a tissue microarray study. Pathology oncology research: POR 16(4):479–484. https://doi.org/10.1007/s12253-010-9270-x
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA (2016) A contemporary prostate Cancer grading system: a validated alternative to the Gleason score. European Urology 69(3):428–435. https://doi.org/10.1016/j.eururo.2015.06.046
Loeb S, Folkvaljon Y, Robinson D, Lissbrant IF, Egevad L, Stattin P (2015) Evaluation of the 2015 Gleason grade groups in a Nationwide population-based cohort. European Urology 69:1135–1141. https://doi.org/10.1016/j.eururo.2015.11.036
Sauter G, Steurer S, Clauditz TS, Krech T, Wittmer C, Lutz F, Lennartz M, Janssen T, Hakimi N, Simon R, von Petersdorff-Campen M, Jacobsen F, von Loga K, Wilczak W, Minner S, Tsourlakis MC, Chirico V, Haese A, Heinzer H, Beyer B, Graefen M, Michl U, Salomon G, Steuber T, Budaus LH, Hekeler E, Malsy-Mink J, Kutzera S, Fraune C, Gobel C, Huland H, Schlomm T (2016) Clinical utility of quantitative Gleason grading in prostate biopsies and prostatectomy specimens. European Urology 69(4):592–598. https://doi.org/10.1016/j.eururo.2015.10.029
Timilshina N, Ouellet V, Alibhai SM, Mes-Masson AM, Delvoye N, Drachenberg D, Finelli A, Jammal MP, Karakiewicz P, Lapointe H, Lattouf JB, Lynch K, Paradis JB, Sitarik P, So A, Saad F (2016) Analysis of active surveillance uptake for low-risk localized prostate cancer in Canada: a Canadian multi-institutional study. World Journal of Urology 35:595–603. https://doi.org/10.1007/s00345-016-1897-0
Meyer CP, Hansen J, Boehm K, Tilki D, Abdollah F, Trinh QD, Fisch M, Sauter G, Graefen M, Huland H, Chun FK, Ahyai SA (2016) Tumor volume improves the long-term prediction of biochemical recurrence-free survival after radical prostatectomy for localized prostate cancer with positive surgical margins. World Journal of Urology 35:199–206. https://doi.org/10.1007/s00345-016-1861-z
Budaus L, Isbarn H, Eichelberg C, Lughezzani G, Sun M, Perrotte P, Chun FK, Salomon G, Steuber T, Kollermann J, Sauter G, Ahyai SA, Zacharias M, Fisch M, Schlomm T, Haese A, Heinzer H, Huland H, Montorsi F, Graefen M, Karakiewicz PI (2010) Biochemical recurrence after radical prostatectomy: multiplicative interaction between surgical margin status and pathological stage. The Journal of Urology 184(4):1341–1346. https://doi.org/10.1016/j.juro.2010.06.018
Isbarn H, Ahyai SA, Chun FK, Budaus L, Schlomm T, Salomon G, Zacharias M, Erbersdobler A, Kollermann J, Sauter G, Huland H, Graefen M, Steuber T (2009) Prevalence of a tertiary Gleason grade and its impact on adverse histopathologic parameters in a contemporary radical prostatectomy series. European Urology 55(2):394–401. https://doi.org/10.1016/j.eururo.2008.08.015
Suardi N, Porter CR, Reuther AM, Walz J, Kodama K, Gibbons RP, Correa R, Montorsi F, Graefen M, Huland H, Klein EA, Karakiewicz PI (2008) A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer 112(6):1254–1263. https://doi.org/10.1002/cncr.23293
Briganti A, Karnes RJ, Joniau S, Boorjian SA, Cozzarini C, Gandaglia G, Hinkelbein W, Haustermans K, Tombal B, Shariat S, Sun M, Karakiewicz PI, Montorsi F, Van Poppel H, Wiegel T (2014) Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. European Urology 66(3):479–486. https://doi.org/10.1016/j.eururo.2013.11.045
Lughezzani G, Briganti A, Karakiewicz PI, Kattan MW, Montorsi F, Shariat SF, Vickers AJ (2010) Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature. European Urology 58(5):687–700. https://doi.org/10.1016/j.eururo.2010.07.034
Lughezzani G, Budaus L, Isbarn H, Sun M, Perrotte P, Haese A, Chun FK, Schlomm T, Steuber T, Heinzer H, Huland H, Montorsi F, Graefen M, Karakiewicz PI (2010) Head-to-head comparison of the three most commonly used preoperative models for prediction of biochemical recurrence after radical prostatectomy. European Urology 57(4):562–568. https://doi.org/10.1016/j.eururo.2009.12.003
Walz J, Chun FK, Klein EA, Reuther A, Saad F, Graefen M, Huland H, Karakiewicz PI (2009) Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. The Journal of Urology 181(2):601–607; discussion 607-608. https://doi.org/10.1016/j.juro.2008.10.033
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading C (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. The American Journal of Surgical Pathology 40(2):244–252. https://doi.org/10.1097/PAS.0000000000000530
Bubendorf L, Nocito A, Moch H, Sauter G (2001) Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. The Journal of Pathology 195(1):72–79. https://doi.org/10.1002/path.893
Rubin MA, Dunn R, Strawderman M, Pienta KJ (2002) Tissue microarray sampling strategy for prostate Cancer biomarker analysis. The American Journal of Surgical Pathology 26(3):312–319. https://doi.org/10.1097/00000478-200203000-00004
Diallo JS, Aldejmah A, Mouhim AF, Fahmy MA, Koumakpayi IH, Sircar K, Begin LR, Mes-Masson AM, Saad F (2008) Co-assessment of cytoplasmic and nuclear androgen receptor location in prostate specimens: potential implications for prostate cancer development and prognosis. BJU International 101(10):1302–1309. https://doi.org/10.1111/j.1464-410X.2008.07514.x
Gannon PO, Lessard L, Stevens LM, Forest V, Begin LR, Minner S, Tennstedt P, Schlomm T, Mes-Masson AM, Saad F (2013) Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer. European Journal of Cancer 49(10):2441–2448. https://doi.org/10.1016/j.ejca.2013.02.026
Koumakpayi IH, Le Page C, Mes-Masson AM, Saad F (2010) Hierarchical clustering of immunohistochemical analysis of the activated ErbB/PI3K/Akt/NF-kappaB signalling pathway and prognostic significance in prostate cancer. British Journal of Cancer 102(7):1163–1173. https://doi.org/10.1038/sj.bjc.6605571
Ross AE, Johnson MH, Yousefi K, Davicioni E, Netto GJ, Marchionni L, Fedor HL, Glavaris S, Choeurng V, Buerki C, Erho N, Lam LL, Humphreys EB, Faraj S, Bezerra SM, Han M, Partin AW, Trock BJ, Schaeffer EM (2016) Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. European Urology 69(1):157–165. https://doi.org/10.1016/j.eururo.2015.05.042
Ruela-de-Sousa RR, Hoekstra E, Hoogland AM, Souza Queiroz KC, Peppelenbosch MP, Stubbs AP, Pelizzaro-Rocha K, van Leenders GJ, Jenster G, Aoyama H, Ferreira CV, Fuhler GM (2016) Low-molecular-weight protein tyrosine phosphatase predicts prostate Cancer outcome by increasing the metastatic potential. European Urology 69(4):710–719. https://doi.org/10.1016/j.eururo.2015.06.040
Belledant A, Hovington H, Garcia L, Caron P, Brisson H, Villeneuve L, Simonyan D, Tetu B, Fradet Y, Lacombe L, Guillemette C, Levesque E (2016) The UGT2B28 sex-steroid inactivation pathway is a regulator of Steroidogenesis and modifies the risk of prostate Cancer progression. European Urology 69(4):601–609. https://doi.org/10.1016/j.eururo.2015.06.054
Bostrom PJ, Bjartell AS, Catto JW, Eggener SE, Lilja H, Loeb S, Schalken J, Schlomm T, Cooperberg MR (2015) Genomic predictors of outcome in prostate Cancer. European Urology 68:1033–1044. https://doi.org/10.1016/j.eururo.2015.04.008
Cullen J, Rosner IL, Brand TC, Zhang N, Tsiatis AC, Moncur J, Ali A, Chen Y, Knezevic D, Maddala T, Lawrence HJ, Febbo PG, Srivastava S, Sesterhenn IA, McLeod DG (2015) A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate Cancer. European Urology 68(1):123–131. https://doi.org/10.1016/j.eururo.2014.11.030
Eggener S (2015) Prostate Cancer screening biomarkers: an emerging embarrassment of 'Riches'? European Urology 70:54–55. https://doi.org/10.1016/j.eururo.2015.09.002
Schlomm T, Weischenfeldt J, Korbel J, Sauter G (2015) The aging prostate is never "normal": implications from the genomic characterization of multifocal prostate cancers. European Urology 68:348–350. https://doi.org/10.1016/j.eururo.2015.04.012
Klein EA, Yousefi K, Haddad Z, Choeurng V, Buerki C, Stephenson AJ, Li J, Kattan MW, Magi-Galluzzi C, Davicioni E (2015) A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. European Urology 67(4):778–786. https://doi.org/10.1016/j.eururo.2014.10.036
Tomlins SA, Day JR, Lonigro RJ, Hovelson DH, Siddiqui J, Kunju LP, Dunn RL, Meyer S, Hodge P, Groskopf J, Wei JT, Chinnaiyan AM (2015) Urine TMPRSS2:ERG plus PCA3 for individualized prostate Cancer risk assessment. European Urology 70:45–53. https://doi.org/10.1016/j.eururo.2015.04.039
Mehra R, Udager AM, Ahearn TU, Cao X, Feng FY, Loda M, Petimar JS, Kantoff P, Mucci LA, Chinnaiyan AM (2015) Overexpression of the long non-coding RNA SChLAP1 independently predicts lethal prostate Cancer. European Urology 70:549–552. https://doi.org/10.1016/j.eururo.2015.12.003
Rubin MA, Girelli G, Demichelis F (2016) Genomic correlates to the newly proposed grading prognostic groups for prostate Cancer. European Urology 69(4):557–560. https://doi.org/10.1016/j.eururo.2015.10.040
Chun FK, Briganti A, Graefen M, Montorsi F, Porter C, Scattoni V, Gallina A, Walz J, Haese A, Steuber T, Erbersdobler A, Schlomm T, Ahyai SA, Currlin E, Valiquette L, Heinzer H, Rigatti P, Huland H, Karakiewicz PI (2007) Development and external validation of an extended 10-core biopsy nomogram. European Urology 52(2):436–444. https://doi.org/10.1016/j.eururo.2006.08.039
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM (2015) Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 313(4):390–397
Acknowledgements
The Network acknowledges contributions to its CPCBN biobank from several Institutions across Canada, namely Centre hospitalier de l’Université de Montreal (CHUM), Centre hospitalier universitaire de Quebec (CHUQ), McGill University Health Centre (MUHC), University Health Network (UHN), University of British Columbia/Vancouver Coastal Health Authority.
Author information
Authors and Affiliations
Contributions
All authors of this research paper have directly participated in the planning, execution, or analysis of the study. All authors of this paper have read and approved the final version submitted. The authors listed below have made substantial contributions to the intellectual content of the paper in the various sections described below:
SR Leyh-Bannurah: data analysis, data management, manuscript writing.
D Trudel: data analysis, protocol development, manuscript writing.
M Latour: project development, manuscript editing.
E Zaffuto: manuscript editing, data analysis.
AA Grosset: manuscript editing, data collection.
C Tam: protocol development, project development, data collection.
V Ouellet: protocol development, project development, manuscript editing.
M Graefen: manuscript editing, data analysis.
L Budäus: manuscript editing, manuscript editing.
A Aprikian: project development, data analysis.
L Lacombe: project development, data analysis.
N Fleshner: data analysis, protocol development, manuscript editing.
ME Gleave: protocol development, data collection.
AM Mes-Masson: protocol development, data collection.
F Saad: project development, data collection, data management, manuscript editing.
PI Karakiewicz: project development, data analysis, manuscript writing, manuscript editing.
Ethics declarations
Conflict of Interest
There are no conflicts of interest. The contents of this manuscript have not been copyrighted or published previously. The contents of this manuscript are not under consideration for publication elsewhere.
Ethical Standars
Informed written consent was obtained and ethical standards were adhered to.
Electronic supplementary material
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Leyh-Bannurah, SR., Trudel, D., Latour, M. et al. A Multi-Institutional Validation of Gleason Score Derived from Tissue Microarray Cores. Pathol. Oncol. Res. 25, 979–986 (2019). https://doi.org/10.1007/s12253-018-0408-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0408-6