Abstract
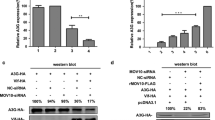
Enterovirus A71 (EV-A71) is the major pathogen responsible for the severe hand, foot and mouth disease worldwide, for which few effective antiviral drugs are presently available. Interferon-α (IFN-α) has been used in antiviral therapy for decades; it has been reported that EV-A71 antagonizes the antiviral activity of IFN-α based on viral 2Apro-mediated reduction of the interferon-alpha receptor 1 (IFNAR1); however, the mechanism remains unknown. Here, we showed a significant increase in IFNAR1 protein induced by IFN-α in RD cells, whereas EV-A71 infection caused obvious down-regulation of the IFNAR1 protein and blockage of IFN-α signaling. Subsequently, we observed that EV-A71 2Apro inhibited IFNAR1 translation by cleavage of the eukaryotic initiation factor 4GI (eIF4GI), without affecting IFNAR1 mRNA levels induced by IFN-α. The inhibition of IFNAR1 translation also occurred in puromycin-induced apoptotic cells when caspase-3 cleaved eIF4GI. Importantly, we verified that 2Apro could activate cellular caspase-3, which was subsequently involved in eIF4GI cleavage mediated by 2Apro. Furthermore, inhibition of caspase-3 activation resulted in the partial restoration of IFNAR1 in cells transfected with 2A or infected with EV-A71, suggesting the pivotal role of both viral 2Apro and caspase-3 activation in the disturbance of IFN-α signaling. Collectively, we elucidate a novel mechanism by which cellular caspase-3 contributes to viral 2Apro-mediated down-regulation of IFNAR1 at the translation level during EV-A71 infection, indicating that caspase-3 inhibition could be a potential complementary strategy to improve clinical anti-EV-A71 therapy with IFN-α.







Similar content being viewed by others
References
Araya RE, Goldszmid RS (2017) IFNAR1 degradation: a new mechanism for tumor immune evasion? Cancer Cell 31:161–163
Castelló A, Alvarez E, Carrasco L (2011) The multifaceted poliovirus 2A protease: regulation of gene expression by picornavirus proteases. J Biomed Biotechnol 2011:369648
Chang YH, Lau KS, Kuo R, Horng JT (2017) dsRNA binding domain of PKR is proteolytically released by enterovirus A71 to facilitate viral replication. Front Cell Infect Microbiol 7:284
Chow KT, Gale M Jr (2015) SnapShot: interferon signaling. Cell 163:1808
Deszcz L, Seipelt J, Vassilieva E, Roetzer A, Kuechler E (2004) Antiviral activity of caspase inhibitors: effect on picornaviral 2A proteinase. FEBS Lett 560:51–55
Duan H, Zhu M, Xiong Q, Wang Y, Xu C, Sun J, Wang C, Zhang H, Xu P, Peng Y (2017) Regulation of enterovirus 2A protease-associated viral IRES activities by the cell’s ERK signaling cascade: implicating ERK as an efficiently antiviral target. Antiviral Res 143:13–21
Fuchs SY (2013) Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J Interferon Cytokine Res 33:211–225
Hsu YY, Liu YN, Lu WW, Kung SH (2009) Visualizing and quantifying the differential cleavages of the eukaryotic translation initiation factors eIF4GI and eIF4GII in the enterovirus-infected cell. Biotechnol Bioeng 104:1142–1152
Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF (1999) Neurologic complications in children with enterovirus 71 infection. N Engl J Med 341:936–942
Huang J, Liao Q, Ooi MH, Cowling BJ, Chang Z, Wu P, Liu F, Li Y, Luo L, Yu S, Yu H, Wei S (2018) Epidemiology of recurrent hand, foot and mouth disease, China, 2008–2015. Emerg Infect Dis 24:432–442
Hung HC, Wang HC, Shih SR, Teng IF, Tseng CP, Hsu JT (2011) Synergistic inhibition of enterovirus 71 replication by interferon and rupintrivir. J Infect Dis 203:1784–1790
Jin Y, Zhang R, Wu W, Duan G (2018) Innate immunity evasion by enteroviruses linked to epidemic hand–foot–mouth disease. Front Microbiol 9:2422
Kuo RL, Kao LT, Lin SJ, Wang RY, Shih SR (2013) MDA5 plays a crucial role in enterovirus 71 RNA-mediated IRF3 activation. PLoS ONE 8:e63431
Lee KM, Chen CJ, Shih SR (2017) Regulation mechanisms of viral IRES-Driven translation. Trends Microbiol 25:546–561
Lee JY, Son M, Kang JH, Choi UY (2018) Serum interleukin-6 levels as an indicator of aseptic meningitis among children with enterovirus 71-induced hand, foot and mouth disease. Postgrad Med 130:258–263
Li Y, Chang Z, Wu P, Liao Q, Liu F, Zheng Y, Luo L, Zhou Y, Chen Q, Yu S, Guo C, Chen Z, Long L, Zhao S, Yang B, Yu H, Cowling BJ (2018) Emerging enteroviruses causing hand, foot and mouth disease, China, 2010–2016. Emerg Infect Dis 24:1902–1906
Liu Y, Zhang Z, Zhao X, Yu R, Zhang X, Wu S, Liu J, Chi X, Song X, Fu L, Yu Y, Hou L, Chen W (2014) Enterovirus 71 inhibits cellular type I interferon signaling by downregulating JAK1 protein expression. Viral Immunol 27:267–276
Lu J, Yi L, Zhao J, Yu J, Chen Y, Lin MC, Kung HF, He ML (2012) Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1. J Virol 86:3767–3776
Marissen WE, Lloyd RE (1998) Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol Cell Biol 18:7565–7574
Morrison JM, Racaniello VR (2009) Proteinase 2Apro is essential for enterovirus replication in type I interferon-treated cells. J Virol 83:4412–4422
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T (2010) Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 9:1097–1105
Plevka P, Perera R, Cardosa J, Kuhn RJ, Rossmann MG (2012) Crystal structure of human enterovirus 71. Science 336:1274
Prévôt D, Darlix JL, Ohlmann T (2003) Conducting the initiation of protein synthesis: the role of eIF4G. Biol Cell 95:141–156
Rasti M, Khanbabaei H, Teimoori A (2019) An update on enterovirus 71 infection and interferon type I response. Rev Med Virol 29:e2016
Sadler AJ, Williams BR (2008) Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559–568
Schmidt NJ, Lennette EH, Ho HH (1974) An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 129:304–309
Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH (2010) Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 10:778–790
Song F, Yu X, Zhong T, Wang Z, Meng X, Li Z, Zhang S, Huo W, Liu X, Zhang Y, Zhang W, Yu J (2018) Caspase-3 inhibition attenuates the cytopathic effects of EV71 infection. Front Microbiol 9:817
Wang C, Zhou R, Zhang Z, Jin Y, Cardona CJ, Xing Z (2015a) Intrinsic apoptosis and proinflammatory cytokines regulated in human astrocytes infected with enterovirus 71. J Gen Virol 96:3010–3022
Wang CY, Huang AC, Hour MJ, Huang SH, Kung SH, Chen CH, Chen IC, Chang YS, Lien JC, Lin CW (2015b) Antiviral potential of a novel compound CW-33 against enterovirus A71 via inhibition of viral 2A protease. Viruses 7:3155–3171
Wang H, Li Y (2019) Recent Progress on Functional Genomics Research of Enterovirus 71. Virol Sin 34:9–21
Wang C, Sun M, Yuan X, Ji L, Jin Y, Cardona CJ, Xing Z (2017) Enterovirus 71 suppresses interferon responses by blocking Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling through inducing karyopherin-α1 degradation. J Biol Chem 292:10262–10274
Yu H, Cowling BJ (2019) Remaining challenges for prevention and control of hand, foot, and mouth disease. Lancet Child Adolesc Health 3:373–374
Yuan J, Shen L, Wu J, Zou X, Gu J, Chen J, Mao L (2018) Enterovirus A71 proteins: structure and function. Front Microbiol 9:286
Zamora M, Marissen WE, Lloyd RE (2002) Multiple eIF4GI-specific protease activities present in uninfected and poliovirus-infected cells. J Virol 76:165–177
Zhang H, Feng H, Luo L, Zhou Q, Luo Z, Peng Y (2010) Distinct effects of knocking down MEK1 and MEK2 on replication of herpes simplex virus type 2. Virus Res 150:22–27
Zhang Y, Li J, Li Q (2018) Immune evasion of enteroviruses under innate immune monitoring. Front Microbiol 9:1866
Zhu M, Duan H, Gao M, Zhang H, Peng Y (2015) Both ERK1 and ERK2 are required for enterovirus 71 (EV71) efficient replication. Viruses 7:1344–1356
Acknowledgements
This work was partially supported by grants from Beijing Natural Science Foundation (No. 19G10290) and National Natural Science Foundation of China (No. 81772184).
Author information
Authors and Affiliations
Contributions
YP and HZ designed the experiments and analyzed the data. BC, YW and SW performed most of the experiments. BC wrote the manuscript. XP, HZ and YP revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Animal and Human Rights Statement
This study does not involve animal experiments.
Rights and permissions
About this article
Cite this article
Chen, B., Wang, Y., Pei, X. et al. Cellular Caspase-3 Contributes to EV-A71 2Apro-Mediated Down-Regulation of IFNAR1 at the Translation Level. Virol. Sin. 35, 64–72 (2020). https://doi.org/10.1007/s12250-019-00151-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-019-00151-y