Abstract
Purpose
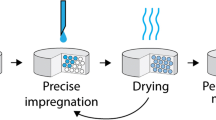
In this study, an active pharmaceutical ingredient (API) was impregnated onto a mesoporous carrier (excipient) in a fluidized bed. Impregnating APIs in porous carriers has the potential to simplify the drug substance development process and leads to the elimination of the blending and granulation unit operations. Impregnation and drying occur simultaneously in fluidized-bed impregnation, and this method precludes several challenges encountered in other impregnation methods. Furthermore, the process ensures a uniform distribution of APIs within the porous carriers. This method also allows for a variety of solvents to be used in the impregnation.
Methods
Using a batch fluidized-bed dryer, impregnation of acetaminophen (APAP) onto a magnesium/aluminum metasilicate (Neusilin R) was studied. Water and methanol were used as transport solvents to impregnate different loadings (w/w) of APAP, namely, ranging from 24 to 0.1%.
Results
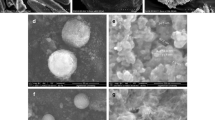
Uniformity tests indicated that APAP loadings of all impregnated products are close to their target loadings across carrier size classes. Tests before and after impregnation showed that there were no changes observed in the physical properties of the carrier, such as particle size, compressibility, and flow properties. X-ray diffraction (XRD) and differential scanning calorimetry (DSC) analysis performed on the samples indicated that the low loadings APAP was present in the carrier pores in its amorphous state. At high loadings, we observed that APAP was mainly present in its amorphous state, however, some degree of crystallinity was observed. Lastly, dissolution tests on the impregnated product showed enhanced dissolution rates in acidic media, and comparable dissolution rate in de-ionized (DI) water, compared to the pure drug crystals.
Conclusion
Our results show that the method of fluidized-bed impregnation yields a product with high uniformity and overcomes several challenges presented by traditional physical blends.

ᅟ









Similar content being viewed by others
References
Mellaerts R, Jammaer JAG, van Speybroeck M, Chen H, Humbeeck JV, Augustijns P, et al. Physical state of poorly water soluble therapeutic molecules loaded into SBA-15 ordered mesoporous silica carriers: a case study with itraconazole and ibuprofen. Langmuir. 2008;24(16):8651–9.
Song SW, Hidajat K, Kawi S. Functionalized SBA-15 materials as carriers for controlled drug delivery: influence of surface properties on matrix-drug interactions. Langmuir. 2005;21(21):9568–75.
Bouledjouidja A, Masmoudi Y, van Speybroeck M, Schueller L, Badens E. Impregnation of fenofibrate on mesoporous silica using supercritical carbon dioxide. Int J Pharm. 2016;499(1–2):1–9.
Kutza C, Metz H, Kutza J, Syrowatka F, Mäder K. Toward a detailed characterization of oil adsorbates as “solid liquids”. Eur J Pharm Biopharm. 2013;84(1):172–82.
Mura P, Valleri M, Cirri M, Mennini N. New solid self-microemulsifying systems to enhance dissolution rate of poorly water soluble drugs. Pharm Dev Technol. 2012;17(3):277–84.
Gupta MK, Vanwert A, Bogner RH. Formation of physically stable amorphous drugs by milling with Neusilin. J Pharm Sci. 2003;92(3):536–51.
Knapik J, Wojnarowska Z, Grzybowska K, Jurkiewicz K, Stankiewicz A, Paluch M. Stabilization of the amorphous ezetimibe drug by confining its dimension. Mol Pharm. 2016;13(4):1308–16.
Grigorov PI, Glasser BJ, Muzzio FJ. Improving dissolution kinetics of pharmaceuticals by fluidized bed impregnation of active pharmaceutical ingredients. AICHE J. 2016;62(12):4201–14.
Lemsi M, Galai H, Louhaichi MR, Fessi H, Kalfat R. Amorphization of atorvastatin calcium by mechanical process: characterization and stabilization within polymeric matrix. J Pharm Innov. 2017;12(3):216–25.
Wong WS, Lee CS, Er HM, Lim WH. Preparation and evaluation of palm oil-based polyesteramide solid dispersion for obtaining improved and targeted dissolution of mefenamic acid. J Pharm Innov. 2017;12(1):76–89.
Ambrogi V, Latterini L, Marmottini F, Tiralti MC, Ricci M. Oxybenzone entrapped in mesoporous silicate MCM-41. J Pharm Innov. 2013;8(4):212–7.
Qian KK, Bogner RH. Application of mesoporous silicon dioxide and silicate in oral amorphous drug delivery systems. J Pharm Sci. 2012;101(2):444–63.
Allgeier MC, Piper JL, Hinds J, Yates MH, Kolodsick KJ, Meury R, et al. Isolation and physical property optimization of an amorphous drug substance utilizing a high surface area magnesium aluminometasilicate (Neusilin((R)) US2). J Pharm Sci. 2016;105(10):3105–14.
Kinoshita M, Baba K, Nagayasu A, Yamabe K, Shimooka T, Takeichi Y’, et al. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J Pharm Sci. 2002;91(2):362–70.
Konno T, Kinuno K, Kataoka K. Physical and chemical changes of medicinals in mixtures with adsorbents in the solid state. I. Effect of vapor pressure of the medicinals on changes in crystalline properties. Chem Pharm Bull. 1986;34(1):301–7.
Qian KK, Suib SL, Bogner RH. Spontaneous crystalline-to-amorphous phase transformation of organic or medicinal compounds in the presence of porous media, part 2: Amorphization capacity and mechanisms of interaction. J Pharm Sci. 2011;100(11):4674–86.
Shen SC, Ng WK, Chia L, Dong YC, Tan RBH. Stabilized amorphous state of ibuprofen by co-spray drying with mesoporous SBA-15 to enhance dissolution properties. J Pharm Sci. 2010;99(4):1997–2007.
Mellaerts R, Mols R, Jammaer JAG, Aerts CA, Annaert P, van Humbeeck J, et al. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur J Pharm Biopharm. 2008;69(1):223–30.
Van Speybroeck M, et al. Ordered mesoporous silica material SBA-15: a broad-spectrum formulation platform for poorly soluble drugs. J Pharm Sci. 2009;98(8):2648–58.
Mallick S, Pattnaik S, Swain K, de PK, Saha A, Ghoshal G, et al. Formation of physically stable amorphous phase of ibuprofen by solid state milling with kaolin. Eur J Pharm Biopharm. 2008;68(2):346–51.
Dhall M, Nanda S, Madan AK. Studies on flash evaporation for preparation of porous solid dispersions using piroxicam as a model drug. J Pharm Innov. 2011;6(4):232–40.
Charnay C, Bégu S, Tourné-Péteilh C, Nicole L, Lerner DA, Devoisselle JM. Inclusion of ibuprofen in mesoporous templated silica: drug loading and release property. Eur J Pharm Biopharm. 2004;57(3):533–40.
Grigorov PI, Glasser BJ, Muzzio FJ. Formulation and manufacture of pharmaceuticals by fluidized-bed impregnation of active pharmaceutical ingredients onto porous carriers. AICHE J. 2013;59(12):4538–52.
Carson JW, Wilms H. Development of an international standard for shear testing. Powder Technol. 2006;167:1–9.
Freeman R. Measuring the flow properties of consolidated, conditioned and aerated powders - a comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007;174(1–2):25–33.
Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60(2):309–19.
Gao Y, Ierapetritou MG, Muzzio FJ. Determination of the confidence interval of the relative standard deviation using convolution. J Pharm Innov. 2013;8(2):72–82.
Oka S, Sahay A, Meng W, Muzzio F. Diminished segregation in continuous powder mixing. Powder Technol. 2017;309:79–88.
Greggy SJ, Sing KSW. Adsorption, Surface Area, and Porosity: Academic Press Inc (London) Ltd; 1982. Appendix.
Dollish FR, Fateley WG, Bentley FF. Characteristic Raman frequencies of organic compounds. New YorkUSA: Wiley; 1974. Appendix One
Diniz JEM, Borges RS, Alves CNA. DFT study for paracetamol and 3,5-disubstituted analogues. J Mol Struct Theochem. 2004;673:93–7.
Hernández B, Pflüger F, Kruglik SG, Ghomi M. Characteristic Raman lines of phenylalanine analyzed by a multiconformational approach. J Raman Spectrosc. 2013;44(6):827–33.
Mellaerts R, Aerts CA, Humbeeck JV, Augustijns P, den Mooter GV, Martens JA. Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials. Chem Commun (Camb). 2007;(13):1375–7.
Shaw LR, Irwin WJ, Grattan TJ, Conway BR. The effect of selected water-soluble excipients on the dissolution of paracetamol and ibuprofen. Drug Dev Ind Pharm. 2005;31(6):515–25.
Bahl D, Bogner RH. Amorphization alone does not account for the enhancement of solubility of drug co-ground with silicate: the case of indomethacin. AAPS PharmSciTech. 2008;9(1):146–53.
Acknowledgements
We like to thank Plamen Grigorov for assistance with some of the experiments. We would like to thank Pavithra Valliappan for the help with the writing of the manuscript.
Funding
Some of the material in this paper is based upon the work supported by the National Science Foundation under Grant Number 1444903.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Rights and permissions
About this article
Cite this article
Omar, T.A., Oka, S., Muzzio, F.J. et al. Manufacturing of Pharmaceuticals by Impregnation of an Active Pharmaceutical Ingredient onto a Mesoporous Carrier: Impact of Solvent and Loading. J Pharm Innov 14, 194–205 (2019). https://doi.org/10.1007/s12247-018-9349-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9349-6