Abstract
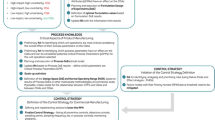
A series of case histories from IQ consortium member companies will be presented to exemplify how the application of the ICH Q11 vision for enhanced Quality by Design (QbD) development of the active pharmaceutical ingredient (API) can lead to differentiated outcomes for elements such as the API supply chain and control strategy, and how changes to such outcomes are managed over the lifecycle. A series of articles will address “flexibility” and look to provide recommendations for the further development of the ICH Q11 vision. The focus of this work will address flexibility associated with the “Enhanced Approaches to the Development of the Control Strategy and Its Implementation in the Manufacturing Process Description.”














Similar content being viewed by others
References
Case Study 1 & 2
ICH Q11 Q9 Guidelines.
Case Study 2
Seibert K, Sethuraman S, Mitchell J, Griffiths K, McGarvey B. The use of routine process capability for the determination of process parameter criticality in small-molecule API synthesis: J. Pharm Innov. 2008;3:105–12.
Mitchell J, Abhinava K, Griffiths K, McGarvey B, Seibert K, Sethuraman S. Unit operations characterization using historical manufacturing performance. Ind Eng Chem Res. 2008;47:6612–21.
Case Study 3
Am Ende D, Clifford P, DeAntonis D, Santamaria C, Brenek S. Preparation of grignard reactants: ftir and calorimetric investigation for safe scale up. Org. Proc. Res. Dev. 1999;3:319–29.
Caygill G, Zanfir M, Gavriilidis A. Scalable reactor design for pharmaceuticals and fine chemicals production. 1: potential scale-up obstacles. Org. Proc. Res. Dev. 2006;10:539–52.
Figueroa I, Vaidyaraman S, Viswanath S. Model-based scale-up and design space determination for a batch reactive distillation with a dean-stark trap. Org. Proc. Res. Dev. 2013;17:1300–10.
Gonzalez-Bobes F, Kopp N, Li L, Deerberg J, Sharma P, Leung S, et al. Scale-up of azide chemistry: a case study. Org. Proc. Res. Dev. 2012;16:2051–7.
Hoekstra L, Vonk P, Hulshof L. Modeling the scale-up of contact drying processes. Org. Proc. Res. Dev. 2006;10(3):409–16.
Tangler A, Szabados E. Overcoming problems at elaboration and scale-up of liquid-phase Pd/C mediated catalytic hydrogenations in pharmaceutical production. Org. Process Res. Dev. 2016;20:1246–51.
Anderson N. Practical use of continuous processing in developing and scaling up laboratory processes. Org. Proc. Res. Dev. 2001;5:613–21.
Johnson M, May S, Calvin J, Remacle J, Stout J, Diseroad W, et al. Development and scale-up of a continuous, high-pressure, asymmetric hydrogenation reaction, workup, and isolation. Org. Proc. Res. Dev. 2012;16:1017–38.
Polster C, Cole K, Burcham C, Campbell B, Frederick A, Hansen M, et al. Pilot-scale continuous production of LY2888721: amide formation and reactive crystallization. Org Proc Res Dev. 2014;18:1295–309.
Zaborenko N, Lynder R, Braden T, Campbell B, Hansen M, Johnson M. Development of pilot-scale continuous production of an LY2886721 starting material by packed-bed hydrogenolysis. Org. Proc. Res. Dev. 2015;19:1231–43.
Food and Drug Administration Code of Federal Regulations, Tittle 21, Volume 4 (21CFR210.3).
Thomson N, Singer R, Seibert K, Luciani C, Srivastava S, Kiesman W, et al. Case studies in development of drug substance control strategy. Org. Proc. Res. Dev. 2015;19:935–48.
Rathore A, Velayudhan A. Guidelines for optimization and scale-up in preparative chromatography. BioPharm International. 2003;16:34–42.
García-Muñoz S, Luciani C, Vaidyaraman S, Seibert K. Definition of design spaces using mechanistic models and geometric projections of probability maps. Org. Proc. Res. Dev. 2015;19:1012–23.
Case Study 5
The development of a control strategy for a final intermediate towards the preparation of a drug substance is described in Org. Process Res.Dev.2016, 20, 1781–1791 (published on October 7, 2016).
Acknowledgements
The authors acknowledge the following for their input and support: Tim Watson, Asher Lower, Tim Curran, Nick Thompson, Steve Tymonko, Jeffrey Kallemeyn, Tim Curran, Adam Looker, John R Donaubauer, and Nathan Ide.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popkin, M.E., Omer, B.A., Seibert, K.D. et al. Part 3: Enhanced Approaches to the Development of the Control Strategy and its Implementation in the Manufacturing Process Description. J Pharm Innov 14, 1–14 (2019). https://doi.org/10.1007/s12247-018-9340-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9340-2