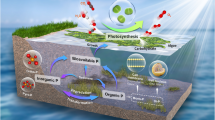
Abstract
Phytoplankton primary production varies considerably with environmental parameters especially in dynamic ecosystems like estuaries. The aim of this study was to investigate short-term primary production along the salinity gradient of a temperate estuary over the course of 1 year. The combination of carbon incorporation and fluorescence methods enabled primary production estimation at short spatial and temporal scales. The electron requirement for carbon fixation was investigated in relation with physical-chemical parameters to accurately estimate primary production at high frequency. These results combined with the variability of the photic layer allowed the annual estimation of primary production along the estuary. Phytoplankton dynamics was closely related to salinity and turbidity gradients, which strongly influenced cells physiology and photoacclimatation. The number of electrons required to fix 1 mol of carbon (C) was ranged between 1.6 and 25 mol electron mol C−1 with a mean annual value of 8 ± 5 mol electron mol C−1. This optimum value suggests that in nutrient replete conditions like estuaries, alternative electron flows are low, while electrons transfer from photosystem II to carbon fixation is highly efficient. A statistical model was used to improve the estimation of primary production from electron transport rate as a function of significant environmental parameters. Based on this model, daily carbon production in the Seine estuary (France) was estimated by considering light and photic zone variability. A mean annual daily primary production of 0.12 ± 0.18 g C m−2 day−1 with a maximum of 1.18 g C m−2 day−1 in summer was estimated which lead to an annual mean of 64.75 g C m−2 year−1. This approach should be applied more frequently in dynamic ecosystems such as estuaries or coastal waters to accurately estimate primary production in those valuable ecosystems.











Similar content being viewed by others
References
Alderkamp, A.-C., H.J.W. de Baar, R.J.W. Visser, and K.R. Arrigo. 2010. Can photoinhibition control phytoplankton abundance in deeply mixed water columns of the Southern Ocean? Limnology and Oceanography 55 (3): 1248–1264. https://doi.org/10.4319/lo.2010.55.3.1248.
Aminot, A., and M. Chaussepied. 1983. Manuel des analyses chimiques en milieu marin, 395. Paris: Editions Jouve, CNEXO.
Aminot, A., and R. Kérouel. 2004. Hydrologie des écosystèmes marins: paramètres et analyses. Méthodes d’analyse en milieu marin, 336. Plouzané: Editions IFREMER.
Aminot, A., and R. Kérouel. 2007. Dosage automatique des nutriments dans les eaux marines: méthodes en flux continu. In Méthodes d’analyse en milieu marin, ed. Ifremer, 188.
Anning, T., H.L. Macintyre, S.M. Pratt, P.J. Sammes, S. Gibb, and R.J. Geider. 2000. Photoacclimation in the marine diatom Skeletonema costatum. Limnology and Oceanography 45 (8): 1807–1817. https://doi.org/10.4319/lo.2000.45.8.1807.
Babin, M., A. Morel, and R. Gagnon. 1994. An incubator designed for extensive and sensitive measurements phytoplankton photosynthetic parameters. Limnology and Oceanography 39: 694–702.
Babin, M., A. Morel, H. Claustre, A. Bricaud, Z. Kolber, and P.G. Falkowski. 1996. Nitrogen- and irradiance-dependent variations of the maximum quantum yield of carbon fixation in eutrophic, mesotrophic and oligotrophic marine systems. Deep Sea Research 43 (8): 1241–1272. https://doi.org/10.1016/0967-0637(96)00058-1.
Bailleul, B., N. Berne, O. Murik, D. Petroutsos, J. Prihoda, A. Tanaka, V. Villanova, R. Bligny, S. Flori, D. Falconet, A. Krieger-liszkay, S. Santabarbara, F. Rappaport, P. Joliot, L. Tirichine, P.G. Falkowski, P. Cardol, C. Bowler, and G. Finazzi. 2015. Mitochondria drives CO 2 assimilation in diatoms. Nature 524 (7565): 366–369. https://doi.org/10.1038/nature14599.
Barranguet, C., and J. Kromkamp. 2000. Estimating primary production rates from photosynthetic electron transport in estuarine microphytobenthos. Marine Ecology Progress Series 204: 39–52. https://doi.org/10.3354/meps204039.
Boynton, W., W. Kemp, and C. Keefe. 1982. A comparative analysis of nutrients and other factors influencing estuarine phytoplankton production. In Estuarine Comparisons, ed. V. S. Kennedy, 69–90. New York: Academic Press.
Brenon, I., and P. Le Hir. 1999. Modelling the turbidity maximum in the Seine estuary (France): identification of formation. Estuarine, Coastal and Shelf Science 49: 525–544.
Campbell, D.A., V. Hurry, A.K. Clarke, P. Gustafsson, and G. Oquist. 1998. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiology and Molecular Biology Reviews: MMBR 62 (3): 667–683.
Chen, C.-T.A., and A.V. Borges. 2009. Reconciling opposing views on carbon cycling in the coastal ocean: continental shelves as sinks and near-shore ecosystems as sources of atmospheric CO2. Deep Sea Research Part II: Topical Studies in Oceanography 56 (8-10): 578–590. https://doi.org/10.1016/j.dsr2.2009.01.001.
Chessel, D., A.B. Dufour, and J. Thioulouse. 2004. The ade4 package - I: one-table methods. R News 4: 5–10.
Claquin, P., I. Probert, S. Lefebvre, and B. Veron. 2008. Effects of temperature on photosynthetic parameters and TEP production in eight species of marine microalgae. Aquatic Microbial Ecology 51: 1–11. https://doi.org/10.3354/ame01187.
Claquin, P., S.N.Í. Longphuirt, P. Fouillaron, P. Huonnic, O. Ragueneau, C. Klein, and A. Leynaert. 2010. Effects of simulated benthic fluxes on phytoplankton dynamic and photosynthetic parameters in a mesocosm experiment (Bay of Brest, France). Estuarine, Coastal and Shelf Science 86 (1): 93–101. https://doi.org/10.1016/j.ecss.2009.10.017.
Cloern, J.E. 1996. Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics 34 (2): 127–168. https://doi.org/10.1029/96RG00986.
Cloern, J.E., S.Q. Foster, and A.E. Kleckner. 2014. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11 (9): 2477–2501. https://doi.org/10.5194/bg-11-2477-2014.
Davison, I.R. 1991. Environmental effects on algal photosynthesis: temperature. Journal of Phycology 27 (1): 2–8. https://doi.org/10.1111/j.0022-3646.1991.00002.x.
Descy, J.-P., F. Darchambeau, T. Lambert, M.P. Stoyneva-Gaertner, S. Bouillon, and A.V. Borges. 2017. Phytoplankton dynamics in the Congo River. Freshwater Biology 62 (1): 87–101.
Dortch, Q., and T.E. Whitledge. 1992. Does nitrogen or silicon limit phytoplankton production in the Mississippi River plume and nearby regions? Continental Shelf Research 12 (11): 1293–1309. https://doi.org/10.1016/0278-4343(92)90065-R.
Dray, S., and A.B. Dufour. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20.
Dubinsky, Z., and N. Stambler. 2009. Photoacclimation processes in phytoplankton: mechanisms, consequences, and applications. Aquatic Microbial Ecology 56: 163–176. https://doi.org/10.3354/ame01345.
Eilers, P.H.C., and J.C.H. Peeters. 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling 42 (3-4): 199–215. https://doi.org/10.1016/0304-3800(88)90057-9.
Endo, T., and K. Asada. 2002. Photosystem I and photoprotection: cyclic electron flow and water-water cycle. In Photoprotection, Photoinhibition, Gene Regulation and Environment, ed. Demmin-Adams, B., Adams, W.W. III, and A.K. Matoo, 205–221. The Netherlands: Springer.
Etcheber, H., A. Taillez, G. Abril, J. Garnier, P. Servais, F. Moatar, and M.V. Commarieu. 2007. Particulate organic carbon in the estuarine turbidity maxima of the Gironde, Loire and Seine estuaries: origin and lability. Hydrobiologia 588 (1): 245–259. https://doi.org/10.1007/s10750-007-0667-9.
Even, S., J.M. Mouchel, P. Servais, N. Flipo, M. Poulin, S. Blanc, M. Chabanel, and C. Paffoni. 2007. Modelling the impacts of combined sewer overflows on the river Seine water quality. Science of the Total Environment 375 (1-3): 140–151. https://doi.org/10.1016/j.scitotenv.2006.12.007.
Falkowski, P.G., and J.A. Raven. 1997. Aquatic photosynthesis. Malden: Blackwell Science.
Garnier, J., G. Billen, J. Némery, and M. Sebilo. 2010. Transformations of nutrients (N, P, Si) in the turbidity maximum zone of the Seine estuary and export to the sea. Estuarine, Coastal and Shelf Science 90 (3): 129–141. https://doi.org/10.1016/j.ecss.2010.07.012.
Genty, B., J. Harbinson, J. Briantais, N.R. Baker, and C. Lane. 1989. The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynthesis Research 25: 249–257.
Goosen, N.K., J. Kromkamp, J. Peene, P. Van Rijswijk, and P. Van Breugel. 1999. Bacterial and phytoplankton production in the maximum turbidity zone of three European estuaries: the Elbe, Westerschelde and Gironde. Journal of Marine Systems 22 (2-3): 151–171. https://doi.org/10.1016/S0924-7963(99)00038-X.
Goss, R., and T. Jakob. 2010. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynthesis Research 106 (1-2): 103–122. https://doi.org/10.1007/s11120-010-9536-x.
Hama, T., T. Miyazaki, Y. Ogawa, T. Iwakuma, M. Takahashi, A. Otsuki, and S. Ichimura. 1983. Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Marine Biology 73 (1): 31–36. https://doi.org/10.1007/BF00396282.
Hancke, K., T. Dalsgaard, M.K. Sejr, S. Markager, and R.N. Glud. 2015. Phytoplankton Productivity in an arctic fjord (West Greenland): estimating electron requirements for carbon fixation and oxygen production. PLoS One 10: 1–23.
Hartig, P., K. Wolfstein, S. Lippemeier, and F. Colijn. 1998. Photosynthetic activity of natural microphytobenthos populations measured by fluorescence (PAM) and 14C-tracer methods: a comparison. Marine Ecology Progress Series 166: 53–62. https://doi.org/10.3354/meps166053.
Hernando, M., I.R. Schloss, G. Malanga, G.O. Almandoz, G.A. Ferreyra, M.B. Aguiar, and S. Puntarulo. 2015. Effects of salinity changes on coastal Antarctic phytoplankton physiology and assemblage composition. Journal of Experimental Marine Biology and Ecology 466: 110–119. https://doi.org/10.1016/j.jembe.2015.02.012.
Holm-Hansen, O., A.F. Amos, and C.D. Hewes. 2000. Reliability of estimating chlorophyll a concentrations in Antarctic waters by measurement of in situ chlorophyll a fluorescence. Marine Ecology Progress Series 196: 103–110. https://doi.org/10.3354/meps196103.
Hunter-Cevera, K.R., M.G. Neubert, R.J. Olson, A.R. Solow, A. Shalapyonok, and H.M. Sosik. 2016. Physiological and ecological drivers of early spring blooms of a coastal phytoplankter. Science 354 (6310): 326–329. https://doi.org/10.1126/science.aaf8536.
Hydes, D., M. Aoyama, A. Aminot, K. Bakker, S. Becker, S. Coverly, A. Daniel, A.G. Dickson, O. Grosso, R. Kerouel, J. van Ooijen, K. Sato, T. Tanhua, E.M.S. Woodward, and J.Z. Zhang. 2010. Determination of dissolved nutrients (N, P, Si) in seawater with high precision and inter-comparability using gas-segmented continuous flow analysers. The GO-SHIP Repeat Hydrography Manual IOCCP Report 134: 1–87.
Johnsen, G., and E. Sakshaug. 2007. Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse-amplitude-modulated and fast-repetition-rate fluorometry 1. Journal of Phycology 43 (6): 1236–1251. https://doi.org/10.1111/j.1529-8817.2007.00422.x.
Johnson, X., and J. Alric. 2013. Central carbon metabolism and electron transport in chlamydomonas reinhardtii: Metabolic constraints for carbon partitioning between oil and starch. Eukaryotic Cell 12 (6): 776–793. https://doi.org/10.1128/EC.00318-12.
Jouenne, F., S. Lefebvre, B. Véron, and Y. Lagadeuc. 2007. Phytoplankton community structure and primary production in small intertidal estuarine-bay ecosystem (eastern English Channel, France). Marine Biology 151 (3): 805–825. https://doi.org/10.1007/s00227-006-0440-z.
Juneau, P., and P. J. Harrison. 2005. Comparison by PAM fluorometry of photosynthetic activity of nine marine phytoplankton grown under identical conditions. photochemistry and photobiology, 649–653.
Kaiblinger, C., and M.T. Dokulil. 2006. Application of fast repetition rate fluorometry to phytoplankton photosynthetic parameters in freshwaters. Photosynthesis Research 88 (1): 19–30. https://doi.org/10.1007/s11120-005-9018-8.
Kimmerer, W.J., A.E. Parker, U.E. Lidström, and E.J. Carpenter. 2012. Short-term and interannual variability in primary production in the low-salinity zone of the San Francisco estuary. Estuaries and Coasts 35 (4): 913–929. https://doi.org/10.1007/s12237-012-9482-2.
Klughammer, C., and U. Schreiber. 2015. Apparent PS II absorption cross-section and estimation of mean PAR in optically thin and dense suspensions of Chlorella. Photosynthesis Research 123 (1): 77–92. https://doi.org/10.1007/s11120-014-0040-6.
Kolber, Z., and P.G. Falkowski. 1993. Use of active fluorescence to estimate phytoplankton photosynthesis in situ. Limnology and Oceanography 38 (8): 1646–1665. https://doi.org/10.4319/lo.1993.38.8.1646.
Kolber, Z.S., O. Prášil, and P.G. Falkowski. 1998. Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochimica et Biophysica Acta - Bioenergetics 1367 (1-3): 88–106. https://doi.org/10.1016/S0005-2728(98)00135-2.
Kromkamp, J.C., and R.M. Forster. 2003. The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. European Journal of Phycology 38 (2): 103–112. https://doi.org/10.1080/0967026031000094094.
Kromkamp, J.C., and J. Peene. 2005. Changes in phytoplankton biomass and primary production between 1991 and 2001 in the Westerschelde estuary (Belgium/The Netherlands). Hydrobiologia 540 (1-3): 117–126. https://doi.org/10.1007/s10750-004-7124-9.
Lawrenz, E., G. Silsbe, E. Capuzzo, P. Ylöstalo, R.M. Forster, S.G.H. Simis, O. Prášil, J.C. Kromkamp, A.E. Hickman, C.M. Moore, M.-H. Forget, R.J. Geider, and D.J. Suggett. 2013. Predicting the electron requirement for carbon fixation in seas and oceans. PLoS One 8 (3): e58137. https://doi.org/10.1371/journal.pone.0058137.
Lionard, M., K. Muylaert, D. Van Gansbeke, and W. Vyverman. 2005. Influence of changes in salinity and light intensity on growth of phytoplankton communities from the Schelde river and estuary (Belgium/The Netherlands). Hydrobiologia 540 (1-3): 105–115. https://doi.org/10.1007/s10750-004-7123-x.
Lohrenz, S.E., G.L. Fahnenstiel, D.G. Redalje, G.A. Lang, M.J. Dagg, T.E. Whitledge, and Q. Dortch. 1999. Nutrients, irradiance, and mixing as factors regulating primary production in coastal waters impacted by the Mississippi River plume. Continental Shelf Research 19 (9): 1113–1141. https://doi.org/10.1016/S0278-4343(99)00012-6.
Lorenzen, C.J. 1966. A method for the continuous measurement of in vivo chlorophyll concentration. Deep Sea Research and Oceanographic Abstracts 13 (2): 223–227. https://doi.org/10.1016/0011-7471(66)91102-8.
Macintyre, H.L., T.M. Kana, T. Anning, and R.J. Geider. 2002. Review Photoacclimatation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. Journal of Phycology 38 (1): 17–38. https://doi.org/10.1046/j.1529-8817.2002.00094.x.
Magnien, R.E., R.M. Summers, and K.G. Sellner. 1992. External nutrient sources, internal nutrient pools, and phytoplankton production in Chesapeake Bay. Estuaries 15 (4): 497. https://doi.org/10.2307/1352393.
Mallin, M.A., H.W. Paerl, J. Rudek, and P.W. Bates. 1993. Regulation of estuarine primary production by watershed rainfall and river flow. Marine Ecology Progress Series 93: 199–203. https://doi.org/10.3354/meps093199.
Masojidek, J., J. Grobbelaar, L. Pechar, and M. Koblizek. 2001. Photosystem II electron transport rates and oxygen production in natural waterblooms of freshwater cyanobacteria during a diel cycle. Journal of Plankton Research 23 (1): 57–66. https://doi.org/10.1093/plankt/23.1.57.
Milligan, A.J., K.H. Halsey, and M.J. Behrenfeld. 2014. HORIZONS: advancing interpretations of 14C-uptake measurements in the context of phytoplankton physiology and ecology. Journal of Plankton Research 37: 692–698.
Morris, E.P., and J.C. Kromkamp. 2003. Influence of temperature on the relationship between oxygen- and fluorescence-based estimates of photosynthetic parameters in a marine benthic diatom (Cylindrotheca closterium). European Journal of Phycology 38 (2): 133–142. https://doi.org/10.1080/0967026031000085832.
Morris, A.W., A.J. Bale, and R.J.M. Howland. 1981. Nutrient distributions in an estuary: Evidence of chemical precipitation of dissolved silicate and phosphate. Estuarine, Coastal and Shelf Science 12 (2): 205–216. https://doi.org/10.1016/S0302-3524(81)80097-7.
Napoléon, C., and P. Claquin. 2012. Multi-parametric relationships between PAM measurements and carbon incorporation, an in situ approach. PLoS One 7: 1–12.
Napoléon, C., V. Raimbault, L. Fiant, P. Riou, S. Lefebvre, L. Lampert, and P. Claquin. 2012. Spatiotemporal dynamics of physicochemical and photosynthetic parameters in the central English Channel. Journal of Sea Research 69: 43–52. https://doi.org/10.1016/j.seares.2012.01.005.
Napoléon, C., L. Fiant, V. Raimbault, and P. Claquin. 2013a. Study of dynamics of phytoplankton and photosynthetic parameters using opportunity ships in the western English Channel. Journal of Marine Systems 128: 146–158. https://doi.org/10.1016/j.jmarsys.2013.04.019.
Napoléon, C., V. Raimbault, and P. Claquin. 2013b. Influence of nutrient stress on the relationships between PAM measurements and carbon incorporation in four phytoplankton species. PLoS One 8 (6): e66423. https://doi.org/10.1371/journal.pone.0066423.
Napoléon, C., L. Fiant, V. Raimbault, P. Riou, and P. Claquin. 2014. Dynamics of phytoplankton diversity structure and primary productivity in the English Channel. Marine Ecology Progress Series 505: 49–64. https://doi.org/10.3354/meps10772.
Némery, J., and J. Garnier. 2007. Origin and fate of phosphorus in the Seine watershed (France): Agricultural and hydrographic P budgets. Journal of Geophysical Research: Biogeosciences 112: 1–14.
Ning, X., D. Vaulot, Z. Liu, and Z. Liu. 1988. Standing stock and production of phytoplankton in the estuary of the Chang-jiang (Yangste River) and the adjacent East China Sea. Marine Ecology Progress Series 49: 141–150. https://doi.org/10.3354/meps049141.
Nixon, S.W. 1995. Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41 (1): 199–219. https://doi.org/10.1080/00785236.1995.10422044.
Nogales, J., S. Gudmundsson, E.M. Knight, B.O. Palsson, and I. Thiele. 2011. Detailing the optimality of photosynthesis in cyanobacteria through systems biology analysis. PNAS 109: 2678–2683.
Pannard, A., P. Claquin, C. Klein, B. Le Roy, and B. Véron. 2008. Short-term variability of the phytoplankton community in coastal ecosystem in response to physical and chemical conditions’ changes. Estuarine, Coastal and Shelf Science 80 (2): 212–224. https://doi.org/10.1016/j.ecss.2008.08.008.
Parizzi, R.A., E. Da, C. Machado, C. Prestes, D. Santos, L.F. Fernandes, M.G. De Camargo, L. Laureno, and M. Jr. 2016. Primary productivity and phytoplankton dynamics in a subtropical estuary: a multiple timescale approach. Scientia Marina 80: 1–13.
Parkhill, J.P., G. Maillet, and J.J. Cullen. 2001. Fluorescence-based maximal quantum yield for PSII as a diagnostic of nutrient stress. Journal of Phycology 37 (4): 517–529. https://doi.org/10.1046/j.1529-8817.2001.037004517.x.
Passy, P., R. Le Gendre, J. Garnier, P. Cugier, J. Callens, F. Paris, G. Billen, P. Riou, and E. Romero. 2016. Eutrophication modelling chain for improved management strategies to prevent algal blooms in the Bay of Seine. Marine Ecology Progress Series 543: 107–125. https://doi.org/10.3354/meps11533.
Pauly, D., and V. Christensen. 1995. Primary production required to sustain global fisheries. 374: 255–257.
Pennock, J., and J. Sharp. 1986. Phytoplankton production in the Delaware Estuary: temporal and spatial variability. Marine Ecology Progress Series 34: 143–155. https://doi.org/10.3354/meps034143.
Sanford, L.P., S.E. Suttles, and J.P. Halka. 2001. Reconsidering the physics of the Chesapeake Bay estuarine turbidity maximum. Estuaries 24 (5): 655. https://doi.org/10.2307/1352874.
Schreiber, U., U. Schliwa, and W. Bilger. 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research 10 (1-2): 51–62. https://doi.org/10.1007/BF00024185.
Schreiber, U., C. Klughammer, and J. Kolbowski. 2012. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynthesis Research 113 (1-3): 127–144. https://doi.org/10.1007/s11120-012-9758-1.
Schuback, N., C. Schallenberg, C. Duckham, M.T. Maldonado, and P.D. Tortell. 2015. Interacting effects of light and iron availability on the coupling of photosynthetic electron transport and CO2-assimilation in marine phytoplankton. PLoS One 10 (7): e0133235. https://doi.org/10.1371/journal.pone.0133235.
Servais, P., and J. Garnier. 2006. Organic carbon and bacterial heterotrophic activity in the maximum turbidity zone of the Seine estuary (France). Aquatic Sciences 68 (1): 78–85. https://doi.org/10.1007/s00027-005-0809-y.
Sferratore, A., J. Garnier, G. Billen, D.J. Conley, and S. Pinault. 2006. Diffuse and point sources of silica in the Seine River Watershed. Environmental Science and Technology 40 (21): 6630–6635. https://doi.org/10.1021/es060710q.
Sharp, J.H., C.H. Culberson, and T.M. Church. 1982. The chemistry of the Delaware estuary. General considerations. Limnology and Oceanography 27 (6): 1015–1028. https://doi.org/10.4319/lo.1982.27.6.1015.
Shaw, P.J., and D.A. Purdie. 2001. Phytoplankton photosynthesis-irradiance parameters in the near-shore UK coastal waters of the North Sea: temporal variation and environmental control. Marine Ecology Progress Series 216: 83–94. https://doi.org/10.3354/meps216083.
Shelly, K., P. Heraud, J. Beardall, and E.T. Al. 2003. NOTE interactive effects of PAR and UV-b radiation on PSII electron transport in the marine alga dunaliella tertiolecta (chlorophyceae ) to better understand the interactions between PAR and UV-B radiation in microalgae. Journal of Phycology 512: 509–512.
Smith, E.M., and W.M. Kemp. 1995. Seasonal and regional variations in plankton community production and respiration for Chesapeake Bay. Marine Ecology Progress Series 116: 217–232. https://doi.org/10.3354/meps116217.
Sorokin, Y.I., and P.Y. Sorokin. 1996. Plankton and primary production in the Lena River estuary and in the south-eastern Laptev Sea. Estuarine, Coastal and Shelf Science 43 (4): 399–418. https://doi.org/10.1006/ecss.1996.0078.
Suggett, D.J., H.L. MacIntyre, and R.J. Geider. 2004. Evaluation of biophysical and optical determinations of light absorption by photosystem II in phytoplankton. Limnology and Oceanography: Methods 2 (10): 316–332. https://doi.org/10.4319/lom.2004.2.316.
Tillmann, U., K.-J. Hesse, and F. Colijn. 2000. Planktonic primary production in the German Wadden Sea. Journal of Plankton Research 22 (7): 1253–1276. https://doi.org/10.1093/plankt/22.7.1253.
Underwood, G.J.C., and J. Kromkamp. 1999. Primary production by phytoplankton and microphytobenthos in estuaries. Advances in Ecological Research 29: 93–153. https://doi.org/10.1016/S0065-2504(08)60192-0.
van Spaendonk, J.C.M., J.C. Kromkamp, and P.R.M. de Visscher. 1993. Primary production of phytoplankton in a turbid coastal plain estuary, the Westerschelde (The Netherlands). Netherlands Journal of Sea Research 31 (3): 267–279. https://doi.org/10.1016/0077-7579(93)90027-P.
Vegter, F. 1977. The closure of the grenvelingen estuary: its influence on phytoplankton primary production and nutrient content. Hydrology 52 (1): 67–71. https://doi.org/10.1007/BF02658083.
Verney, R., R. Lafite, and J.C. Brun-Cottan. 2009. Flocculation potential of estuarine particles: The importance of environmental factors and of the spatial and seasonal variability of suspended particulate matter. Estuaries and Coasts 32 (4): 678–693. https://doi.org/10.1007/s12237-009-9160-1.
Wang, Z.B., M.C.J.L. Jeuken, H. Gerritsen, H.J. De Vriend, and B.A. Kornman. 2002. Morphology and asymmetry of the vertical tide in the Westerschelde estuary. Continental Shelf Research 22 (17): 2599–2609. https://doi.org/10.1016/S0278-4343(02)00134-6.
Zhu, Y., J. Ishizaka, S.C. Tripathy, S. Wang, Y. Mino, T. Matsuno, and D.J. Suggett. 2016. Variation of the photosynthetic electron transfer rate and electron requirement for daily net carbon fixation in Ariake Bay, Japan. Journal of Oceanography 72 (5): 761–776. https://doi.org/10.1007/s10872-016-0370-4.
Acknowledgements
The authors wish to thank those who participated in the sampling campaigns and in the evening sampling treatments, especially Matthieu Filoche, Guillaume Izabel, and the technical staff of the CREC—marine station of Luc-sur-Mer. Authors also want to thank Laurent Perez for his implication in the conception of sampling device.
Funding
This work was support by the GIP Seine-Aval project “PROUESSE.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Deana Erdner
Electronic supplementary material
ESM 1
(DOCX 350 kb)
Rights and permissions
About this article
Cite this article
Morelle, J., Schapira, M., Orvain, F. et al. Annual Phytoplankton Primary Production Estimation in a Temperate Estuary by Coupling PAM and Carbon Incorporation Methods. Estuaries and Coasts 41, 1337–1355 (2018). https://doi.org/10.1007/s12237-018-0369-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0369-8