Abstract
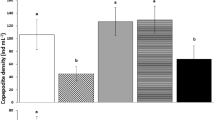
Frequent blooms of the dinoflagellate Alexandrium catenella in southern Chile encouraged undertaking the present study which uses the oyster Ostrea chilensis as a model for evaluating the feeding, growth, lipid storage and mortality responses to diets containing paralytic shellfish poisoning (PSP) produced by A. catenella. Medium-term (30 days) physiological responses of two groups of juvenile oysters were measured every 10 days. Five replicates were exposed to diets containing A. catenella and other five replicates were fed with a diet containing the non-toxic algae Isochrysis galbana. Diets were continuously supplied at a concentration of 2 mg L−1, in which the feeding and metabolic activity was measured, and the scope for growth calculated. Lipids storage, actual growth and mortality were also measured every 10 days. The results showed that the toxic diet has dramatic negative effects on feeding and metabolism of the juvenile individuals of O. chilensis, with high reduction of the lipid storage and growth. Mortality was also increased in individuals fed with the contaminated diet. This study supports the conclusion that the toxic dinoflagellate A. catenella restricts the energy acquisition in the juvenile O. chilensis, an important fishery and aquaculture resource in southern Chile.







Similar content being viewed by others
References
Anderson, D.M. 1989. A global perspective in Red Tides: biology, environmental science and toxicology. In Toxic algal blooms and red tides, ed. T. Okaichi, D.M. Anderson, and T. Nemoto, 11–16. New York: Elsevier.
Anderson, D.M., D.M. Kulis, G.J. Doucette, J.C. Gallager, and E. Balech. 1994. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeast United States and Canada as determined by morphology, bioluminescence, toxin composition, and mating compatibility. Marine Biology 120: 467–478.
Bardouil, M., M. Bohec, M. Cormerais, S. Bougrier, and P. Lassus. 1993. Experimental study of the effects of a toxic microalgae diet on feeding of the oyster Crassostrea gigas (Thunberg). Journal of Shellfish Research. 12: 417–422.
Basti, L., S. Nagai, J. Go, S. Okano, K. Nagai, T. Watanabe, T. Suzuki, and Y. Tanaka. 2015. Differential inimical effects of Alexandrium spp. and Karenia spp. on cleavage, hatching and two larval stages of Japanese pearl oyster Pinctada fucata martensii. Harmful Algae 43: 1–12.
Bricelj, V., A. Cembella, D. Laby, S. Shumway, and T. Cucci. 1996. Harmful and toxic algal blooms. In Comparative physiological and behavioral responses to PSP toxins in two bivalve molluscs, the softshell clam, Mya arenaria, and surfclam Spisula solidissima, ed. T. Yasumoto, Y. Oshima, and Y. Fukuyo, 405–408. Japan: UNESCO.
Bricelj, V.M., L. Connell, K. Konoki, S.P. MacQuarrie, T. Scheuer, W.A. Catterall, and V.L. Trainer. 2005. Sodium channel mutation leading to saxitoxin resistance in clams increase risk of PSP. Nature 434: 763–767.
Bricelj, V.M., S.P. MacQuarrie, J.A.E. Doane, and L.B. Connell. 2010. Evidence of selection for resistance to paralytic shellfish toxins during the early life history of soft-shell clam (Mya arenaria) populations. Limnology and Oceanography 55: 2463–2475.
Caers, M., P. Coutteau, and P. Sorgeloos. 1999. Dietary impact of algal and artificial diets, fed at different feeding rations, on the growth and fatty acid composition of Tapes philippinarum (L.) spat. Aquaculture 170: 307–322.
Chaparro, O.R., Y.A. Montiel, C.J. Segura, V.M. Cubillos, R.J. Thompson, and J.M. Navarro. 2008a. The effect of salinity on clearance rate in the suspension-feeding estuarine gastropod Crepipatella dilatata under natural and controlled conditions. Estuarine, Coastal Shelf Science 76: 861–868.
Chaparro, O.R., C.J. Segura, Y.A. Montiel, R.J. Thompson, and J.M. Navarro. 2008b. Variations in the quantity and composition of seston from an estuary in southern Chile on different temporal scales. Estuarine, Coastal Shelf Science 76: 845–860.
Chen, C.Y., and H.N. Chou. 1998. Transmission of the paralytic shellfish poisoning toxins, from dinoflagellate to gastropod. Toxicon 36: 515–522.
Clément, A., A. Aguilera, and C. Fuentes. 2002. Análisis de Marea Roja en el Archipiélago de Chiloé, Contingencia 2002. Resúmenes XXII Jornadas de Ciencias del Mar 126 p.
Colin, S.P., and H.G. Dam. 2007. Comparison of the functional and numerical responses of resistant versus non-resistant populations of the copepod Acartia hudsonica fed the toxic dinoflagellate Alexandrium tamarense. Harmful Algae 6: 875–882.
Conover, R.J. 1966. Assimilation of organic matter by zooplankton. Limnology and Oceanography 11: 338–345.
Coughlan, J. 1969. The estimation of filtering rate from the clearance of suspensions. Marine Biology 2: 356–358.
Cucci, T.L., S.E. Shumway, R.C. Newell, and C.M. Yentsch. 1985. Toxic dinoflagellates. In A preliminary study of the effects of Gonyaulax tamarensis on feeding in bivalve molluscs, ed. D.M. Anderson, A.W. White, and D.G. Baden, 395–400. New York: Elsevier.
Dam, H.G. 2013. Evolutionary adaptation of marine zooplankton to global change. Annual Review Marine Science 5: 349–370.
Davis, H.C., and A. Calabrese. 1964. Combined effects of temperature and salinity on development of eggs and growth of larvae of M. mercenaria and C. virginica. United States Fish and Wildlife Service. Fishery Bulletin 63: 643–655.
Dupuy, J.L., and A.K. Sparks. 1968. Gonyaulax washingtonensis, its relationship to Mytilus californianus and Crassostrea gigas as a source of paralytic shellfish toxin in Sequim Bay, Washington. Proceedings of the National Shellfish Association 58: 2.
Elliott, J.M., and W. Davison. 1975. Energy equivalents of oxygen consumption in animal energetics. Oecologia (Berl.) 19: 195–201.
Emlet, R.B., and S.S. Sadro. 2006. Linking stages of life history: how larval quality translates into juvenile performance for an intertidal barnacle (Balanus glandula). Integrative & Comparative Biology 46: 334–346.
Fernández-Reiriz, M.J., J.M. Navarro, A.M. Contreras, and U. Labarta. 2008. Trophic interactions between the toxic dinoflagellate Alexandrium catenella and Mytilus chilensis: feeding and digestive behaviour to long-term exposure. Aquatic Toxicology 87: 245–251.
Fernández-Reiriz, M.J., J.M. Navarro, B.A. Cisternas, U. Labarta, and J.M.F. Babarro. 2013. Enzymatic digestive activity and absorption efficiency in Tagelus dombeii upon Alexandrium catenella exposure. Helgoland Marine Research 67: 653–661.
Gainey, L.F., and S.E. Shumway. 1988. A compendium of the responses of bivalve mollusc to toxic dinoflagellates. Journal of Shellfish Research. 7: 623–628.
Gobler, C.J., D.J. Lonsdale, and G.L. Boyer. 2005. A synthesis and review of causes and impact of harmful brown tide blooms caused by the alga, Aureococcus anophagefferens. Estuaries 28: 726–749.
Guillard, R.R.L. 1975. Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals, ed. W.L. Smith and M.H. Chanley, 29–60. N.Y: Plenum Press.
Guillard, R.R.L. 1995. Culture methods. In Manual on harmful marine microalgae. IOC Manuals and Guides, ed. G.M. Hallegraeff, D.M. Anderson, and A.D. Cembella, 45–62. Paris: No33 UNESCO.
Guzmán, L., I. Campodonico, and J. Hermosilla. 1975. Estudio sobre un florecimiento tóxico causado por Gonyaulax catenella en Magallanes. I.- Distribución espacial y temporal de G. catenella, vol. 6, 173–183. Punta Arenas: Anales del Instituto de la Patagonia.
Haberkorn, H., C. Lambert, N. Le Goïc, J. Moal, M. Suquet, M. Guéguen, I. Sunila, and P. Soudant. 2010. Effects of Alexandrium minutum exposure on nutrition-related processes and reproductive output in oysters Crassostrea gigas. Harmful Algae. 9: 427–439.
Hall, J.M., C.C. Parrish, and R.J. Thompson. 2002. Eicosapentaenoic acid regulates scallop (Placopecten magellanicus) membrane fluidity in response to cold. The Biology Bulletin 202: 201–203.
Lassus, P., R. Baron, P. Garen, P. Truquet, P. Masselin, M. Bardouil, D. Leguay, and Z. Amzil. 2004. Paralytic shellfish poison outbreaks in the Penzé estuary: environmental factors affecting toxin uptake in the oyster, Crassostrea gigas. Aquatic Living Resources 17: 207–214.
Le Goïc, N., H. Hégaret, C. Fabioux, P. Miner, M. Suquet, C. Lambert, and P. Soudant. 2013. Impact of the toxic dinoflagellate Alexandrium catenella on Pacific oyster reproductive output: application of flow cytometry assays on spermatozoa. Aquatic Living Resources 26: 221–228.
Lesser, M.P., and S.E. Shumway. 1993. Effects of toxic dinoflagellates on clearance rates and survival in juvenile bivalve molluscs. Journal of Shellfish Research 12: 377–381.
Leverone, J.R., S.E. Shumway, and N.J. Blake. 2007. Comparative effects of the toxic dinoflagellate Karenia brevis on clearance rates in juveniles of four bivalve mollusks from Florida, USA. Toxicon 49: 634–645.
Li, S.C., W.X. Wang, and D.P.F. Hsieh. 2002. Effects of toxic dinoflagellate Alexandrium tamarense on the energy budgets and growth of two marine bivalves. Marine and Environmental Research 53: 145–160.
Long, A.R., S.J. Massie, and W.J. Tyznik. 1988. Rapid direct extraction derivatization method for the determination of acylglycerol lipids in selected sample materials. Journal Food Science 53: 940–959.
Marsden, I.D., and S.E. Shumway. 1992. Effects of dinoflagellate Alexandrium tamarense on the greenshell mussel, Perna canaliculus. New Zealand Journal of Marine and Freshwater Research 26: 371–378.
Marsden, I.D., and S.E. Shumway. 1993. The effect of a toxic dinoflagellate (Alexandrium tamarense) on the oxygen uptake of juvenile filter-feeding bivalve molluscs. Comparative Biochemistry and Physiology A 106: 769–773.
May, S.P., J.A.M. Burkholder, S.E. Shumway, G.H. Wikfors, D. Frank, M. Hsia, H. Hégaret, and Q. Dorch. 2010. Effects of the toxic dinoflagellate Alexandrium monilatum on survival, grazing and behavioral response of three ecologically important shellfish species. Harmful Algae 9: 281–293.
McLusky, D.S.1981. The estuarine ecosystem. 2° edition, Blackie, Glasgow, Chapter 2. Primary producers. pp. 37–52.
Molinet, C., A. Lafon, G. Lembeye, and C. Moreno. 2003. Patrones de distribución espacial y temporal de floraciones de Alexandrium catenella (Whedon & Kofoid) Balech 1985, en agua interiores de la patagonia noroccidental de Chile. Revista Chilena de Historia Natural. 76: 681–698.
Montory, J.A., O.R. Chaparro, J.A. Pechenik, C.M. Diederich, and V.M. Cubillos. 2014. Impact of short-term salinity stress on larval development of the marine gastropod Crepipatella fecunda (Calyptraeidae). Journal of Experimental Marine Biology and Ecology 458: 39–45.
Mu, C., and Q. Li. 2013. Effects of the dinoflagellate Alexandrium catenella on the early development of the Pacific oyster Crassostrea gigas. Journal Shellfish Research 32: 689–694.
Navarro, J.M., and A.M. Contreras. 2010. An integrative response by Mytilus chilensis to the toxic dinoflagellate Alexandrium catenella. Marine Biology 157: 1967–1974.
Navarro, J.M., and C.M. González. 1998. Physiological responses of the Chilean scallop Argopecten purpuratus to decreasing salinities. Aquaculture 167: 315–327.
Navarro, J.M., K. González, B. Cisternas, J.A. López, O.R. Chaparro, C.J. Segura, M. Córdova, B. Suárez-Isla, M.J. Fernández-Reiriz, and U. Labarta. 2014. Contrasting physiological responses of two populations of the razor clam Tagelus dombeii with different histories of exposure to paralytic shellfish poisoning (PSP). PLoS One 9: e105794.
Padilla, D.K., M.H. Doall, C.J. Gobler, A. Hartson, and K. O’Boyle. 2006. Brown tide alga, Aureococcus anophagefferens, can affect growth but not survivorship of Mercenaria mercenarialarvae. Harmful Algae 5: 736–748.
Przeslawski, R., P.E. Bourdeau, M.H. Doall, J. Pan, L. Perino, and D.K. Padilla. 2008. The effects of a harmful alga on bivalve larval lipid stores. Harmful Algae 7: 802–807.
Sernapesca. 2013. Anuario estadístico de pesca. Valparaíso: Servicio Nacional de Pesca. Available at http://www.sernapesca.cl.
Sewell, M.A. 2005. Utilization of lipids during early development of the sea urchin Evenchinus chloroticus. Marine Ecology Progress Series 304: 133–142.
Shumway, S.E., T.L. Cucci, R.C. Newell, and C.M. Yentsch. 1985. Particle selection, ingestion and absorption in filter-feeding bivalves. Journal of Experimental Marine Biology and Ecology 91: 77–92.
Sinesky, M. 1974. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proceedings of the National Academy of Science. USA 71: 522–525.
Solís, I.F. 1967. Observaciones biológicas en ostras (Ostrea chilensis Philippi) de Pullinque. Biología Pesquera. Chile 2: 51–82.
Soudant, P., Y. Marty, J. Moal, and J. Samain. 1996. Fatty acids and egg quality in great scallop. Aquaculture International 4: 191–200.
Tran, D., A. Ciutat, A. Mat, J. Massabuau, H. Hégaret, C. Lambert, N. LeGoic, and P. Soudant. 2015. The toxic dinoflagellate Alexandrium minutum disrupts daily rhythmic activities at gene transcription, physiological and behavioral levels in the oyster Crassostrea gigas. Aquatic Toxicology 158: 4–49.
Vanderplog, H.A., J.R. Liebig, and A.A. Gluck. 1996. Evaluation of different phytoplankton for supporting development of zebra mussel larvae (Dreissena polymorpha): the importance of size and polyunsaturated fatty acid content. Journal Great Lakes Research 22: 36–45.
Wacker, A., and E. von Elert. 2002. Strong influences of larval diet history on subsequent post-settlement growth in the freshwater mollusc Dreissena polymorpha. Proceedings of the Royal Society of London. B. Biological Science 269: 2113–2119.
Warkentin, M., H.M. Freese, U. Karsten, and R. Schumann. 2007. New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots. Applied and Environmental Microbiology 73: 6722–6729.
Widdows, J. 1985. Physiological procedures. In The effects of stress and pollution on marine animals, ed. B.L. Bayne, D.A. Brown, K. Burns, D.R. Dixon, A. Ivanoci, D.R. Livingstone, D.M. Lowe, M.N. Moore, A.R.D. Steinberg, and J. Widdows, 161–178. New York: Praeger Scientific Publications.
Yan, T., M. Zhou, M. Fu, R. Yu, Y. Wang, and J. Li. 2003. Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture 217: 167–178.
Acknowledgments
The authors are grateful to Elizabeth Encalada and Samyra Aliste for their valuable assistance during the experiments. This study was funded by the Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT-CHILE), by research grant to JMN (FONDECYT 1120470).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marco Bartoli
Rights and permissions
About this article
Cite this article
Navarro, J.M., Labraña, W., Chaparro, O.R. et al. Physiological Constraints in Juvenile Ostrea chilensis Fed the Toxic Dinoflagellate Alexandrium catenella . Estuaries and Coasts 39, 1133–1141 (2016). https://doi.org/10.1007/s12237-015-0061-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-0061-1