Abstract
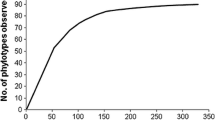
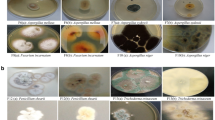
This is the first exhaustive report on the fungal community biodiversity in hypersaline water in România. A total of 27 fungal strains (19 molds and eight yeast) have been isolated from Lopătari hypersaline water, Buzau County. Based on classical investigation, these strains have been identified as belonging to the genera Aureobasidium, Alternaria, Aspergillus, Penicillium, and Fusarium. The molecular characterization of fungal isolates at species level was performed using PCR-RFLP analysis of the 5.8S-ITS region. PCR products were digested with different combinations of endonucleases. The most frequently isolated species were Aspergillus niger (14.81% of all isolates), A. versicolor, (14.81%) and Penicillium crustosum (14.81%). In addition, ribosomal restriction patterns which exhibited profiles specific to Aureobasidium pullulans were derived, and to discriminate between Aureobasidium isolates, the elongase-encoding gene (ELO) was chosen as a genetic marker followed by digestion with endonuclease HhaI. Five yeast isolates displayed restriction patterns corresponding to Aureobasidium melanogenum (18.52%) and three isolates to Aureobasidium pullulans (11.11%). In addition, the RFLP types of Aureobasidium pullulans varieties with HhaI are clearly distinguished and could be applied to assess the intraspecific variability.




Similar content being viewed by others
References
Buchalo AS, Nevo E, Wasser SP, Oren A, Molitoris HP (1998) Fungal life in the extremely hypersaline water of the Dead Sea: first records. Proc R Soc Lond Ser B Biol Sci 265(1404):1461–1465. https://doi.org/10.1098/rspb.1998.0458
Butinar L, Santos S, Spencer-Martins I, Oren A, Gunde-Cimerman N (2005) Yeast diversity in hypersaline habitats. FEMS Microbiol Lett 244(2):229–234. https://doi.org/10.1016/j.femsle.2005.01.043
Butinar L, Frisvad JC, Gunde-Cimerman N (2011) Hypersaline waters – a potential source of foodborne toxigenic aspergilli and penicillia. FEMS Microbiol Ecol 77:186–199. https://doi.org/10.1111/j.1574-6941.2011.01108.x
Cantrell SA, Casillas-Martínez L, Molina M (2006) Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol Res 110(8):962–970. https://doi.org/10.1016/j.mycres.2006.06.005
Cantrell SA, Baez-Félix C (2010) Fungal molecular diversity of a Puerto Rican subtropical hypersaline microbial mat. Fungal Ecol 3: 402–405. 10.1016/j.funeco.2010.04.001
Cojoc R, Merciu S, Popescu G, Dumitru L, Kamekura M, Enache M (2009) Extracellular hydrolytic enzymes of halophilic bacteria isolated from a subterranean rock salt crystal. Rom Biotechnol Lett 14:4658–4664
De Curtis F, Caputo L, Castoria R, Lima G, Stea G, De Cicco V (2004) Use of fluorescent amplified fragment length polymorphism (fAFLP) to identify specific molecular markers for the biocontrol agent Aureobasidium pullulans strain LS30. Postharvest Biol Technol 34:179–186. https://doi.org/10.1016/j.postharvbio.2004.05.008
Diguta CF, Vincent B, Guilloux-Benatier M, Alexandre H, Rousseaux S (2011) PCR ITS-RFLP: a useful method for identifying fungal isolates on grapes. Food Microbiol 28(6):1145–1154. https://doi.org/10.1016/j.fm.2011.03.006
Enache M, Itoh T, Kamekura M, Teodosiu G, Dumitru L (2007) Haloferax prahovense sp. nov., an extremely halophilic archaeon isolated from a Romanian salt lake. Int J Syst Evol Microbiol 57(2):393–397. https://doi.org/10.1099/ijs.0.64674-0
Enache M, Itoh T, Kamekura M, Popescu G, Dumitru L (2008a) Halophilic archaea isolated from man-made young (200 years) salt lakes in Slănic, Prahova, Romania. Cent Eur J Biol 3(4):388–395
Enache M, Itoh T, Kamekura M, Popescu G, Dumitru L (2008b) Halophilic archaea of Haloferax genus isolated from anthropocentric Telega (Palada) salt lake. Proc Rom Acad, Series B 10(1–2):11–16
Enache M, Popescu G, Dumitru L, Kamekura M (2009) The effect of Na+/Mg2+ ratio on the amylase activity of haloarchaea isolated from Techirghiol lake, Romania, a low salt environment. Proc Rom Acad Series B 1:3–7
Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A (1999) Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int J Syst Bacteriol 49:329–337. https://doi.org/10.1099/00207713-49-1-329
Garabito MJ, Marques MC, Ventosa A (1998). Halotolerant Bacillus diversity in hypersaline enviroments. Can J Microbiol 44:95–102. https://doi.org/10.1139/w97-125
Gostinčar C, Turk M, Trbuha T, Vaupotič T, Plemenitaš A, Gunde-Cimerman N (2008) Expression of fatty-acid-modifying enzymes in the halotolerant black yeast Aureobasidium pullulans (de Bary) G. Arnaud under salt stress. Stud Mycol 61:51–59. https://doi.org/10.3114/sim.2008.61.04
Gostinčar C, Ohm RA, Kogej T, Sonjak S, Turk M, Zajc J, Zalar P, Grube M, Sun H, Han J, Sharma A, Chiniquy J, Ngan CY, Lipzen A, Barry K, Grigoriev IV, Gunde-Cimerman N (2014) Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genomics 15(549):1471–2164. https://doi.org/10.1186/1471-2164-15-549
Grant WD (2004) Life at low water activity. Philos Trans R Soc Lond Ser B Biol Sci 359(1448):1249–1267. https://doi.org/10.1098/rstb.2004.1502
Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitaš A (2000) Hypersaline waters in salterns - natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32:235–240. https://doi.org/10.1111/j.1574-6941.2000.tb00716.x
Gunde-Cimerman N, Butinar L, Sonjak S, Plemenitas A (2005) Halotolerant and halophilic fungi from coastal environments in the Arctics. In book: adaptation to life at high salt concentrations in archaea, bacteria, and eukarya, 397–423. doi:https://doi.org/10.1007/1-4020-3633-7_26
Gunde-Cimerman N, Ramos J, Plemenitaš A (2009) Halotolerant and halophilic fungi. Mycol Res 113(11):1231–1241. https://doi.org/10.1016/j.mycres.2009.09.002
Liu J, Liu Z, Chi Z, Zhang L, Zhang D (2009) Intraspecific diversity of Aureobasidium pullulans strains from different marine environments. J Ocean Univ China 8(3):241–246
Lodder J (1974) The yeasts; a taxonomic study. North Holland Publishing Co., Amsterdam
Loncaric I, Donat C, Antlinger B, Oberlerchner JT, Heissenberger B, Moosbeckhofer R (2008) Strain-specific detection of two Aureobasidium pullulans strains, fungal biocontrol agents of fire blight by new, developed multiplex-PCR. J Appl Microbiol 104(5):1433–1441. https://doi.org/10.1111/j.1365-2672.2007.03668.x
Martínez-Culebras PV, Ramón D (2007) An ITS-RFLP method to identify black Aspergillus isolates responsible for OTA contamination in grapes and wine. Int J Food Microbiol 113:147–153. https://doi.org/10.1016/j.ijfoodmicro.2006.06.023
Nagahama T, Hamamoto M, Nakase T, Horikoshi K (2001). Rhodotorula lamellibrachii sp. nov., a new yeast species from a tubeworm collected at the deep-sea floor in Sagami Bay and its phylogenetic analysis. Antonie Leeuwenhoek, 80:317–323. https://doi.org/10.1023/A:1013043301388
Nazareth S, Gonsalves V, Nayak S (2012) A first record of obligate halophilic aspergilli from the dead sea. Indian J Microbiol 52(1):22–27. https://doi.org/10.1007/s12088-011-0225-z
Nazareth S, Gonsalves V (2014) Halophilic Aspergillus penicillioides from athalassohaline, thalassohaline, and polyhaline environments. Front Microbiol 5(412):1–5. https://doi.org/10.3389/fmicb.2014.00412
Neagu S, Enache M, Cojoc R (2014) Extracellular hydrolytic activities of halophilic microorganisms isolated from Balta Albă salt lake. Rom Biotechnol Lett 19(1):8951–8958
Pitt JI, Hocking D (2009) Fungi and food spoilage. https://doi.org/10.1007/978-0-387-92207-2
Proca GI, Matei F, Diguta CF, Jurcoane S (2017) Salt tolerance of bacterial strains isolated from hypersaline water located in Lopatari, Romania. Scientific Bulletin. Series F. Biotechnologies, vol. XXI, 229–232
Schena L, Ippolito A, Zahavi T, Cohen L, Nigro F, Droby S (1999) Genetic diversity and biocontrol activity of Aureobasidium pullulans isolates against postharvest rots. Postharvest Biol Technol 17:189–199. https://doi.org/10.1016/S0925-5214(99)00036-8
Sepcic K, Zalar P, Gunde-Cimerman N (2010) Low water activity induces the production of bioactive metabolites in halophilic and halotolerant fungi. Marine drugs 9(1):43–58. https://doi.org/10.3390/md9010043
Urzì C, De Leo F, Lo Passo C, Criseo G (1999) Intra-specific diversity of Aureobasidium pullulans strains isolated from rocks and other habitats assessed by physiological methods and by random amplified polymorphic DNA (RAPD). J Microbiol Methods 36:95–105
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Ventosa A (2006) Unusual microorganisms from unusual habitats: hypersaline environments. In: Logan NA, Lappin-Scott NH, Oyston PCF (eds) Prokayotic diversity - mechanism and significance. Cambridge University Press, Cambridge, pp 223–253
Yurlova NA, Uijthof JM, de Hoog GS (1996) Distinction of species in Aureobasidium and related genera by PCR-ribotyping. Antonie Van Leeuwenhoek 69:323–329
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungi ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Ninsky JJ, White TJ (eds) PCR protocols. A guide to methods and applications. Academic Press, San Diego, pp 315–322
Zalar P, De Hoog GS, Gunde-Cimerman N (1999) Ecology of halotolerant dothideaceous black yeasts. Stud Mycol:38–48
Zalar P, Gostinčar C, de Hoog GS, Uršič V, Sudhadham M, Gunde-Cimerman N (2008) Redefinition of Aureobasidium pullulans and its varieties. Stud Mycol 61:21–38. https://doi.org/10.3114/sim.2008.61.02
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Diguță, C.F., Proca, I.G., Jurcoane, Ș. et al. Molecular characterization by PCR-RFLP of indigenous fungal isolates from hypersaline stream water in România. Folia Microbiol 64, 407–414 (2019). https://doi.org/10.1007/s12223-018-0664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-018-0664-6