Abstract
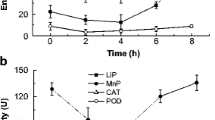
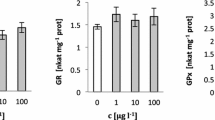
In this study, the effect of chloramine T (Chl-T) on the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GR) and glutathione S-transferase (GST); the levels of reduced (GSH) and oxidised glutathione (GSSG) and their ratios; and also membrane lipid peroxidation (LPO) levels in Phanerochaete chrysosporium were investigated in a dose- (0.25–1 mmol/L) and time-dependent (1.5–9 h) manner. The highest SOD activity was observed in 0.5 mmol/L Chl-T at 6th hour as 1.48-fold of its control. The observed highest level in CAT activities was 4.6-fold of control in 0.5 and 0.75 mmol/L at the 6th hour. The GSH levels that were over the control showed decreasing tendency from the beginning of incubation, except 0.25 mmol/L. In contrast with GSH level variations, GSSG levels reached 10.0-fold of its control by showing increasing tendency with the increases in concentration and time. While the GSH/GSSG ratios were over the control at 0.25 mmol/L during all incubation, they fell under the control values at the earlier hours of incubation with the increasing concentrations of Chl-T. Glutathione-related enzymes GSH-Px, GR and GST were also induced with Chl-T treatment, and the highest activities were 3.29-, 7.5- and 6.56-fold of their controls, respectively. On the other hand, the increases in LPO levels with increasing concentration and time up to 5.27-fold of its control showed that the inductions observed in antioxidant system could not prevent the Chl-T-based oxidative stress.









Similar content being viewed by others
References
Aebi H (1974) Catalase. In: Bergmeyer HV (ed) Methods in enzymatic analysis, vol 2. Academic Press, New York, pp 674–684
Beever RE, Bollard EG (1990) The nature of the stimulation of fungal growth by potato extract. J Gen Microbiol 60:273–279
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bullock GL, Herman RL, Waggy C (1991) Hatchery efficacy trials with chloramine-T for control of bacterial gill disease. J Aquat Anim Health 3(1):48–50. https://doi.org/10.1577/1548-8667(1991)003<0048:HETWCT>2.3.CO;2
Crosti N, Servidei T, Bajer J, Serra A (1987) Modification of the 6-hydroxydopamine technique for the correct determination of superoxide dismutase. J Clin Chein Clin Biochem 25:265–266
Deavall DG, Martin EA, Horner JM, Roberts R (2012) Drug-induced oxidative stress and toxicity. J Toxicol 2012:1–13. https://doi.org/10.1155/2012/645460
Debuf YM (1991) The veterinary formulary handbook of medicines used in veterinary practice. Pharmaceutical Press
Dieckhaus CM, Roller SG, Santos WL, Sofia RD, Macdonald TL (2001) Role of glutathione S-transferases A1-1, M1-1, and P1-1 in the detoxification of 2-phenylpropenal, a reactive felbamate metabolite. Chem Res Toxicol 14(5):511–516. https://doi.org/10.1021/tx000141e
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879. https://doi.org/10.1016/S0899-9007(02)00916-4
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239–247. https://doi.org/10.1038/35041687
Fortuniak A, Zadzinski R, Bilinski T, Bartosz G (1996) Glutathione depletion in the yeast Saccharomyces cerevisiae. Biochem Mol Biol Int 38(5):901–910
Gao H, Liu C (2014) Biochemical and morphological alteration of listeria monocytogenes under environmental stress caused by chloramine-T and sodium hypochlorite. Food Control 46:455–461. https://doi.org/10.1016/j.foodcont.2014.05.016
Ghanem G, Legros F, Lejeune F, Frühling J (1982) Comparison and evaluation of different methods for α-MSH labelling. J Immunol Methods 54(2):223–232. https://doi.org/10.1016/0022-1759(82)90063-1
Gray MJ, Wholey WY, Jakob U (2013) Bacterial responses to reactive chlorine species. Annu Rev Microbiol 67(1):141–160. https://doi.org/10.1146/annurev-micro-102912-142520
Habig WH, Pabst M, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Halliwell B (2006) Reactive species and antioxidants redox biology is a fundamental theme of aerobic life. Plant Physiol 141(2):312–322. https://doi.org/10.1104/pp.106.077073
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defences. Int J Mol Sci 13(3):3145–3175. https://doi.org/10.3390/ijms13033145
Kang JI Jr, Neidigh JW (2008) Hypochlorous acid damages histone proteins forming 3-chlorotyrosine and 3, 5-dichlorotyrosine. Chem Res Toxicol 21(5):1028–1038. https://doi.org/10.1021/tx7003486
Kayali HA, Tarhan L (2004) The effect of cultural conditions on the variations of SOD, CAT and GSH-Px activities and LPO levels in the filamentous fungus Fusarium equiseti. Turk J Chem 28(2):213–222
Kujala VM, Reijula KE, Ruotsalainen EM, Heikkinen K (1995) Occupational asthma due to chloramine-T solution. Respir Med 89(10):693–695. https://doi.org/10.1016/0954-6111(95)90137-X
Marzinzik AL, Sharpless KB (2001) A simple method for the preparation of N-Sulfonylsulfilimines from sulfides. J Org Chem 66(2):594–596. https://doi.org/10.1021/jo0012039
Massey V, Williams CH (1965) On the reaction mechanism of yeast glutathione reductase. J Biol Chem 240(11):4470–4480
Nagy P, Ashby MT (2007) Reactive sulfur species kinetics and mechanisms of the oxidation of cysteine by hypohalous acid to give cysteine sulfenic acid. J Am Chem Soc 129(45):14082–14091. https://doi.org/10.1021/ja0737218
Ngo EO, Nutter LM (1994) Status of glutathione and glutathione-metabolizing enzymes in menadione-resistant human cancer cells. Biochem Pharmacol 47(2):421–424. https://doi.org/10.1016/0006-2952(94)90036-1
Ogino T, Packer L, Maguire JJ (1997) Neutrophil antioxidant capacity during the respiratory burst loss of glutathione induced by chloramines. Free Radic Biol Med 23(3):445–452. https://doi.org/10.1016/S0891-5849(97)00115-9
Owen JB, Butterfield DA (2010) Measurement of oxidized/reduced glutathione ratio. Methods Mol Biol 648:269–277
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Powell MD, Speare DJ, Wright GM (1995) Morphological changes in rainbow trout (Oncorhynchus mykiss) gill epithelia following repeated intermittent exposure to chloramine-T. Can J Zool 73(1):154–165. https://doi.org/10.1139/z95-018
Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32(3):329–342. https://doi.org/10.1046/j.1365-313X.2002.01427.x
Röhrdanz E, Schmuck G, Ohler S, Kahl R (2001) The influence of oxidative stress on catalase and MnSOD gene transcription in astrocytes. Brain Res 900(1):128–136. https://doi.org/10.1016/S0006-8993(01)02277-6
Russell A (1998) Microbial susceptibility and resistance to chemical and physical agents. In: Topley and Wilson’s microbiology and microbial infections. John Wiley & Sons, Inc
Sanchez JG, Speare DJ, Johnson GJ (1997) Morphometric and histochemical assessment of the branchial tissue response of rainbow trout, Oncorhynchus mykiss (Walbaum), associated with chloramine-T treatment. J Fish Dis 20(5):375–381. https://doi.org/10.1046/j.1365-2761.1997.00312.x
Schemedes A, Holmer GA (1989) New thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J Am Oil Chem Soc 66(6):813–817. https://doi.org/10.1007/BF02653674
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27(9):916–921. https://doi.org/10.1016/S0891-5849(99)00177-X
Spickett CM, Jerlich A, Panasenko OM, Arnhold J, Pitt AR, Stelmaszyñska T, Schaur RJ (2000) The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim Pol 47(4):889–900
Stief TW (2003) The physiology and pharmacology of singlet oxygen. Med Hypotheses 60(4):567–572. https://doi.org/10.1016/S0306-9877(03)00026-4
Teare JP, Punchard NA, Powell JJ, Lumb PJ, Mitchell WD, Thompson RP (1993) Automated spectrophotometric method for determining oxidized and reduced glutathione in liver. Clin Chem 39(4):686–689
Tien M, Kirk TK (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci U S A 81(8):2280–2284. https://doi.org/10.1073/pnas.81.8.2280
Tongul B, Tarhan L (2014) The effect of menadione-induced oxidative stress on the in vivo reactive oxygen species and antioxidant response system of Phanerochaete chrysosporium. Process Biochem 49(2):195–202. https://doi.org/10.1016/j.procbio.2013.11.004
Vallis KA, Wolf CR (1996) Relationship between the adaptive response to oxidants and stable menadione-resistance in Chinese hamster ovary cell lines. Carcinogenesis 17(4):649–654. https://doi.org/10.1093/carcin/17.4.649
Videla LA (2009) Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol 1(1):72–78. https://doi.org/10.4254/wjh.v1.i1.72
Wang HF, Zhong XH, Shi WY, Guo B (2013) Study of malondialdehyde (MDA) content, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in chickens infected with avian infectious bronchitis virus. Afr J Biotechnol 10(45):9213–9217
Witko-Sarsat V, Nguyen AT, Descamps-Latscha B (1993) Immunomodulatory role of phagocyte-derived chloramines involving lymphocyte glutathione. Mediat Inflamm 2(3):235–241. https://doi.org/10.1155/S0962935193000328
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tongul, B., Kavakcıoğlu, B. & Tarhan, L. Chloramine T induced oxidative stress and the response of antioxidant system in Phanerochaete chrysosporium . Folia Microbiol 63, 325–333 (2018). https://doi.org/10.1007/s12223-017-0571-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0571-2