Abstract
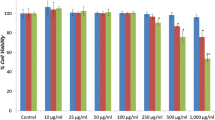
Many lichen secondary metabolites contributed to the field of pharmacology as an active ingredient of different drugs for years. In the present study, we aimed to test the anticancer activity of olivetoric acid (OA), which we isolated from Pseudevernia furfuracea (L.) Zopf in human hepatocellular carcinoma cells (HepG2). In addition, we used non-tumoral human liver cells (THLE2) to test the level of side effects of OA in vitro. For this purpose, cytotoxic (apoptotic and necrotic), oxidant, genotoxic activities and expression levels of apoptotic genes caused by different concentrations (12.5–400 mg/L) of OA were tested on both cells. Flow cytometric and cytotoxicity tests (MTT and LDH) revealed that OA (100–400 mg/L) had a higher rate of apoptotic effects on HepG2 cells compared to THLE2. Total oxidative stress and oxidative DNA damage levels caused by all concentrations of OA on HepG2 cells was significantly (p < 0.05) higher compared to negative control. Trials with concentrations of 100–400 mg/L significantly (p < 0.05) increased total antioxidant capacity on THLE2 cells compared to the control group. As a result, based on human hepatocellular carcinoma, it is hoped that OA may contribute to the combined or alternative treatment process.










Similar content being viewed by others
References
Ahmad N, Fatima N, Ahmad I, Anis M (2015) Effect of PGRs in adventitious root culture in vitro: present scenario and future prospects. Rend Fis Acc Lincei 26:307–321
Alam P, Kamaluddin S-E et al (2016) The effect of over-expression of rate limiting enzymes on the yield of artemisinin in Artemisia annua. Rend Fis Acc Lincei 27:311–319
Asahina Y, Shibata S (1971) Chemistry of lichen substances. Japan Society for the Promotion of Science, Tokyo
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16:273–284
Basile A, Rigano D, Loppi S et al (2015) Antiproliferative, antibacterial and antifungal activity of the lichen Xanthoria parietina and its secondary metabolite parietin. Int J Mol Sci 16:7861–7875
Bessadóttir M, Eiríksson FF, Becker S et al (2015) Anti-proliferative and pro-apoptotic effects of lichen-derived compound protolichesterinic acid are not mediated by its lipoxygenase-inhibitory activity. Prostaglandins Leukot Essent Fatty Acids 98:39–47
Brisdelli F, Perilli M, Sellitri D et al (2016) Protolichesterinic acid enhances doxorubicin-induced apoptosis in HeLa cells in vitro. Life Sci 158:89–97
Culberson CF (1969) Chemical and botanical guide to lichen products. The University of North Carolina Press, Chapel Hill
Dinçsoy AB, Cansaran Duman D (2017) Changes in apoptosis-related gene expression profiles in cancer cell lines exposed to usnic acid lichen secondary metabolite. Turk J Biol 41:484–493
Ebrahim HY, Elsayed HE, Mohyeldin MM et al (2016) Norstictic acid inhibits breast cancer cell proliferation, migration, invasion, and in vivo invasive growth through targeting c-met. Phyther Res 30:557–566
El Zawawy N, El Shafay S, Abomohra AE-F (2020) Macroalgal activity against fungal urinary tract infections: in vitro screening and evaluation study. Rend Fis Acc Lincei 31:165–175
El-Darier SM, El-Ahwany AMD, Elkenany ET, Abdeldaim AA (2018) An in vitro study on antimicrobial and anticancer potentiality of thyme and clove oils. Rend Fis Acc Lincei 29:131–139
Emsen B, Aslan A, Togar B, Turkez H (2016) In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm Biol 54:1748–1762
Emsen B, Turkez H, Togar B, Aslan A (2017) Evaluation of antioxidant and cytotoxic effects of olivetoric and physodic acid in cultured human amnion fibroblasts. Hum Exp Toxicol 36:376–385
Emsen B, Aslan A, Turkez H et al (2018a) The anti-cancer efficacies of diffractaic, lobaric, and usnic acid: in vitro inhibition of glioma. J Cancer Res Ther 14:941–951
Emsen B, Togar B, Turkez H, Aslan A (2018b) Effects of two lichen acids isolated from Pseudevernia furfuracea (L.) Zopf in cultured human lymphocytes. Zeitschrift fur Naturforsch Sect C A J Biosci 73:303–312
Emsen B, Sadi G, Bostanci A, Aslan A (2020) In vitro evaluation of cytotoxic, oxidative, genotoxic, and apoptotic activities of physodic acid from Pseudevernia furfuracea in HepG2 and THLE2 cells. Plant Biosyst. https://doi.org/10.1080/11263504.2020.1852329
Fernández-Moriano C, Divakar PK, Crespo A, Gómez-Serranillos MP (2017) In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol Appl Pharmacol 316:83–94
Gnutzmann D, Kortes N, Sumkauskaite M et al (2018) Transvascular therapy of hepatocellular carcinoma (HCC), status and developments. Minim Invasive Ther Allied Technol 27:69–80
Goga M, Elečko J, Marcinčinová M et al (2020) Lichen metabolites: an overview of some secondary metabolites and their biological potential. Co-evolution of secondary metabolites. Springer, Cham, pp 175–209
Gómez-Lechón MJ, Donato MT, Castell JV, Jover R (2003) Human hepatocytes as a tool for studying toxicity and drug metabolism. Curr Drug Metab 4:292–312
Hong J-M, Suh S-S, Kim TK et al (2018) Anti-cancer activity of lobaric acid and lobarstin extracted from the antarctic lichen Stereocaulon alpnum. Molecules 23:658
Ijaz S, Akhtar N, Khan MS et al (2018) Plant derived anticancer agents: a green approach towards skin cancers. Biomed Pharmacother 103:1643–1651
Kaakati R, Zhao R, Bao X et al (2019) Non-apoptotic roles of caspases in stem cell biology, carcinogenesis, and radiotherapy. Curr Stem Cell Rep 5:31–37
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kiliç N, Derici MK, Büyük I et al (2018) Evaluation of in vitro anticancer activity of vulpinic acid and its apoptotic potential using gene expression and protein analysis. Indian J Pharm Educ Res 52:626–634
Kılıç N, Aras S, Cansaran-Duman D (2018) Determination of vulpinic acid effect on apoptosis and mRNA expression levels in breast cancer cell lines. Anticancer Agents Med Chem 18:2032–2041
Kim H, Kim KK, Hur J-S (2015) Anticancer activity of lichen metabolites and their mechanisms at the molecular level. Recent advances in lichenology. Springer India, New Delhi, pp 201–208
Koparal AT, Ulus G, Zeytinoğlu M et al (2010) Angiogenesis inhibition by a lichen compound olivetoric acid. Phyther Res 24:754–758
Kountouras J, Zavos C, Chatzopoulos D (2005) Apoptotic and anti-angiogenic strategies in liver and gastrointestinal malignancies. J Surg Oncol 90:249–259
Kumar K, Mishra JPN, Singh RP (2020) Usnic acid induces apoptosis in human gastric cancer cells through ROS generation and DNA damage and causes up-regulation of DNA-PKcs and γ-H2A.X phosphorylation. Chem Biol Interact 315:108898
Kunliang L, Zhicheng J, Xiaolong H et al (2020) A biodegradable multifunctional porous microsphere composed of carrageenan for promoting imageable trans-arterial chemoembolization. Int J Biol Macromol 142:866–878
Lai KP, Cheung A, Ho CH et al (2020) Transcriptomic analysis reveals the oncogenic role of S6K1 in hepatocellular carcinoma. J Cancer 11:2645–2655
Lim B, Greer Y, Lipkowitz S, Takebe N (2019) Novel apoptosis-inducing agents for the treatment of cancer, a new arsenal in the toolbox. Cancers (Basel) 11:1087
Maiwall R, Sharma MK (2014) Hepatitis B virus infection and hepatocellular carcinoma. In: Cancer-causing viruses and their inhibitors, pp 121–155
Masubuchi Y (2006) Metabolic and non-metabolic factors determining troglitazone hepatotoxicity: a review. Drug Metab Pharmacokinet 21:347–356
Minicis SD, Marzioni M, Benedetti A, Svegliati-Baroni G (2013) New insights in hepatocellular carcinoma: from bench to bedside. Ann Transl Med 1:15
Mitrović T, Stamenković S, Cvetković V et al (2014) Platismatia glauca and Pseudevernia furfuracea lichens as sources of antioxidant, antimicrobial and antibiofilm agents. EXCLI J 13:938–953
Molnár K, Farkas E (2010) Current results on biological activities of lichen secondary metabolites: a review. Zeitschrift fur Naturforsch Sect C J Biosci 65:157–173
Nguyen TT, Yoon S, Yang Y et al (2014) Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PLoS ONE 9:e111575
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for anticancer therapy. Int J Mol Sci 19:448
Poor MHS, Khatami M, Azizi H, Abazari Y (2017) Cytotoxic activity of biosynthesized Ag nanoparticles by Plantago major towards a human breast cancer cell line. Rend Fis Acc Lincei 28:693–699
Purvis OW, Coppins BJ, Hawksworth DL et al (1992) The lichen flora of Great Britain and Ireland. Natural History Museum Publications in Association with the British Lichen Society, London
Qi W, Lu C, Huang H et al (2020) (+)-usnic acid induces ROS-dependent apoptosis via inhibition of mitochondria respiratory chain complexes and Nrf2 expression in lung squamous cell carcinoma. Int J Mol Sci 21:876
Radha G, Raghavan SC (2017) BCL2: a promising cancer therapeutic target. Biochim Biophys Acta Rev Cancer 1868:309–314
Ranković B, Kosanić M (2019) Lichens as a potential source of bioactive secondary metabolites. In: Ranković B (ed) Lichen secondary metabolites. Springer, Cham, pp 1–29
Raoul J-L, Forner A, Bolondi L et al (2019) Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev 72:28–36
Russo A, Piovano M, Lombardo L et al (2006) Pannarin inhibits cell growth and induces cell death in human prostate carcinoma DU-145 cells. Anticancer Drugs 17:1163–1169
Šeklić DS, Obradović AD, Stanković MS et al (2018) Proapoptotic and antimigratory effects of Pseudevernia furfuracea and Platismatia glauca on colon cancer cell lines. Food Technol Biotechnol 56:421–430
Sharma D, Parveen K, Oza A, Ledwani L (2018) Synthesis of anthraquinone-capped TiO2 nanoparticles using R. emodi roots: preparation, characterization and cytotoxic potential. Rend Fis Acc Lincei 29:649–658
Solárová Z, Liskova A, Samec M et al (2020) Anticancer potential of lichens’ secondary metabolites. Biomolecules 10:87
Suh S-S, Kim TK, Kim JE et al (2017) Anticancer activity of ramalin, a secondary metabolite from the antarctic lichen Ramalina terebrata, against colorectal cancer cells. Molecules 22:1361
Sun Y, Ma W, Yang Y et al (2019) Cancer nanotechnology: enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy. Asian J Pharm Sci 14:581–594
Tanman Ü, Yangın S, Cansaran-Duman D (2020) Determination of dysregulated mirna expression levels by qrt-pcr after the application of usnic acid to breast cancer. Anticancer Agents Med Chem 20:548–558
Tas I, Yildirim AB, Ozkan E et al (2019) Biological evaluation and phytochemical profiling of some lichen species. Acta Aliment 48:457–465
Taş İ, Han J, Park S-Y et al (2019) Physciosporin suppresses the proliferation, motility and tumourigenesis of colorectal cancer cells. Phytomedicine 56:10–20
Tomović J, Kosanić M, Ranković B et al (2019) Phytochemical analysis and biological activity of extracts of lichen Physcia semipinnata: as a new source of pharmacologically active compounds. Farmacia 67:346–353
Turk H, Yilmaz M, Tay T et al (2006) Antimicrobial activity of extracts of chemical races of the lichen Pseudevernia furfuracea and their physodic acid, chloroatranorin, atranorin, and olivetoric acid constituents. Zeitschrift fur Naturforsch Sect C J Biosci 61:499–507
Wege H, Li J, Ittrich H (2019) Treatment lines in hepatocellular carcinoma. Visc Med 35:266–272
Wirth V (1995) Die Flechten Baden Württembergs. Ulmer, Stuttgart
Yang Y, Nguyen TT, Pereira I et al (2019) Lichen secondary metabolite physciosporin decreases the stemness potential of colorectal cancer cells. Biomolecules 9:797
Yurdacan B, Egeli U, Eskiler GG et al (2019) The role of usnic acid-induced apoptosis and autophagy in hepatocellular carcinoma. Hum Exp Toxicol 38:201–215
Zhao Y, Wang M, Xu B (2020) A comprehensive review on secondary metabolites and health-promoting effects of edible lichen. J Funct Foods. https://doi.org/10.1016/j.jff.2020.104283
Funding
This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) [Grant Number 117Z632].
Author information
Authors and Affiliations
Contributions
BE and GS designed the experiments. All authors carried out the experiments. BE and GS analysed the data. BE wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors confirmed manuscript publication.
Ethical approval
Our research needed no ethics approval regarding the experiment.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Emsen, B., Sadi, G., Bostanci, A. et al. Evaluation of the biological activities of olivetoric acid, a lichen-derived molecule, in human hepatocellular carcinoma cells. Rend. Fis. Acc. Lincei 32, 135–148 (2021). https://doi.org/10.1007/s12210-021-00976-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-021-00976-4