Abstract
In this study, a new zirconium-mediated cycloaddition for preparing dibenzosilole derivatives was developed using silicon-bridged diynes and electron-withdrawing alkynes as starting materials. The preparation of silicon-bridged diynes from 1-bromide-2-iodobenzene, terminal alkynes, and dimethyldichlorosilane was also studied. Unlike in the previous synthesis methods, much higher yields of electron-withdrawing group-substituted dibenzosilole derivatives were obtained. In addition, a new synthesis strategy for preparing benzonaphthosilole derivatives using internal alkynes, 1,4-dibromobenzene, and electron-withdrawing alkynes as starting materials is proposed. Compared with previous methods, alkyl, phenyl, and electron-withdrawing groups can be successfully introduced onto aromatic rings, and the positions of these substituents can be easily controlled. The cycloaddition reactions for dibenzosilole and benzonaphthosilole derivatives are highly efficient one-pot processes, and the raw materials are available and easily prepared. Using these new methods, a series of novel multi-substituted dibenzonsilole and benzonaphthosilole derivatives were obtained effectively.
Similar content being viewed by others
Introduction
Silole derivatives have attracted considerable attention in recent years because of their σ*–π* conjugation and aggregation-induced emission (AIE) [1] properties. The σ*–π* conjugation decreases the lowest unoccupied molecular orbital energy level, endowing silole with good electron acceptance ability [2]. As AIE molecules, silole derivatives are promising candidates for electron-transporting and light-emitting layers in optoelectronic devices [3, 4].
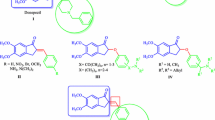
Dibenzosilole molecules have received much attention because of their applications as important building blocks of different donor units and new conjugated polymers for solar cells [5,6,7], electroluminescence materials [8], and explosives detection [9]. Many researchers have adopted the ring-closure method [10,11,12,13,14,15] for preparing dibenzosilole derivatives. However, this method has some limitations such as the difficulties in introducing substituents onto benzene rings and preparing raw materials. Matsuda et al. [16] reported an iridium(I)-catalyzed cycloaddition method for synthesizing dibenzosilole derivatives (Scheme 1), in which electron-donating groups were introduced onto benzene rings. Unfortunately, the reaction was sluggish when R3 was a dimethoxycarbonyl group (an electron-withdrawing group, EWG), and the product could hardly be obtained (yield = 7%). Interestingly, we found that silicon-bridged diynes can react with Cp2ZrBu2 and then undergo a cycloaddition reaction to yield 79% of dimethoxycarbonyl-substituted dibenzosilole derivative. In addition, various silicon-bridged diynes were subjected to a zirconium-mediated cycloaddition reaction to yield 64–79% of novel alkoxycarbonyl-substituted dibenzosilole derivatives (Scheme 1).
The synthesis of benzonaphthosilole (benzo[b]silafluorene) derivatives has seldom been reported although they are promising silole derivatives with extended π-conjugation based on dibenzosilole (Fig. 1) [17,18,19], and no synthesis of benzonaphthosilole derivatives has been reported where alkyl, phenyl, and EWGs groups were substituted on the aromatic rings. Because substituents can effectively regulate molecular properties, lack of a synthesis method has limited the performance studies of benzonaphthosilole derivatives. Fortunately, a multi-substituted benzonaphthosilole derivative (Fig. 1) is obtained by our strategy, which combines zirconium-mediated coupling, Sonogashira coupling, and the novel zirconium-mediated cycloaddition in moderate yield. Using this strategy, propyl, phenyl, and dimethoxycarbonyl groups can be efficiently and selectively introduced onto aromatic rings.
In our previous studies, zirconacyclopentadienes have been used as intermediates for the synthesis of naphthacene derivatives, multi-substituted benzenes, and 1,2,3,4-tetraalkyl-1,4-diarylbutadienes [20,21,22]. In these studies, zirconacyclopentadienes were formed using silicon-free terminal alkynes and internal alkynes as starting materials. However, in this study, silicon-containing acetylenes, i.e., silicon-bridged diynes, were used as the starting materials for zirconium-mediated cycloaddition. A series of dibenzosilole and benzonaphthosilole derivatives were efficiently synthesized from silicon-bridged diynes by one-pot processes. The zirconium-mediated cycloaddition showed good group tolerance for silicon-bridged diynes having different alkyl and aryl groups.
Experimental Details
Reagents and Measurements
Anhydrous tetrahydrofuran (THF) was distilled from sodium and benzophenone under dry nitrogen. Catalysts PdCl2(PPh3)2 and Pd(PPh3)4 were purchased from Woerjiming, China, and n-butyllithium (n-BuLi) was purchased from Ouhechem, China. All other chemicals and reagents were purchased from TCI (Japan) and Aladdin (China) and were used without further purification.
All reactions were carried out under nitrogen protection except for special instructions. 1H and 13C NMR spectra were recorded on a Bruker AVANCE III spectrometer (Switzerland) in deuterated chloroform using tetramethylsilane as the internal standard. Mass spectra were obtained on an LCQ Deca XP MAX (Thermo Fisher, USA) mass spectrometer system. Thin-layer chromatography (TLC) was performed using Energy GF254 plates.
Synthesis of Alkoxycarbonyl-Substituted Dibenzosilole Derivatives
The preparation of alkoxycarbonyl-substituted dibenzosilole derivatives is shown in Scheme 2. Phenyl, pentyl, and alkoxycarbonyl-substituted dibenzosilole derivatives were synthesized from terminal alkynes and 1-bromo-2-iodobenzene.
General Preparation Procedure of 2-Alkynylbromobenzene (2)
First, 316 mg (0.45 mmol) of bis(triphenylphosphine)palladium dichloride (PdCl2(PPh3)2) and 95 mg (0.5 mmol) of cuprous iodide (CuI) were added to 100 mL of triethylamine, and the mixture was stirred. Then, 4.24 g (1.9 mL, 15 mmol) of 1-bromo-2-iodobenzene and 15 mmol of alkynes (1) were added, and the mixture was further stirred overnight at room temperature. The mixture was filtered, and the filtrate was evaporated under a reduced pressure. The residue was purified by flash column chromatography on silica gel with n-hexane as eluent to obtain 2-alkynylbromobenzene.
Spectroscopic data
1-bromo-2-(phenylethynyl)benzene ( 2a ) [ 23 ]:
1H NMR (400 MHz, CDCl3): δ 7.65–7.56 (m, 4H), 7.38 (dd, J = 4.9, 1.9 Hz, 3H), 7.31 (td, J = 7.6, 1.2 Hz, 1H), 7.19 (td, J = 7.8, 1.7 Hz, 1H)
1-bromo-2-(pentylethynyl)benzene ( 2b ) [ 24 ]:
1H NMR (400 MHz, CDCl3): δ 7.54 (dd, J = 8.0, 1.3 Hz, 1H), 7.41 (dt, J = 7.7, 1.3 Hz, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 7.10 (td, J = 7.7, 1.4 Hz, 1H), 2.45 (t, J = 7.0 Hz, 2H), 1.64 (p, J = 7.1 Hz, 2H), 1.52–1.43 (m, 2H), 1.36 (h, J = 7.2 Hz, 2H), 0.95–0.89 (m, 3H)
General Preparation Procedure for Silicon-Bridged Diynes (5)
First, 6.96 mL (2.5 mol/L, 17.4 mmol) of n-BuLi was added dropwise to a solution of 2 (15 mmol) in 35 mL of THF at − 78 °C, and the mixture was stirred for 1 h. Then, 7.1 mL of dimethyldichlorosilane (Me2SiCl2) in 3 mL of THF (cooled to − 78 °C first) was added to the mixture in one portion, and the obtained mixture was gradually warmed to room temperature. After stirring for 18 h, the reaction was quenched with n-hexane, the mixture was filtrated, and the filtrate was evaporated under a reduced pressure to give crude 1-chlorodimethylsily-2-alkynylbenzene (3), which was then dissolved in 3 mL of THF and protected under nitrogen. The solution of 3 was used directly in the next step without further purification. The quenching, filtration, and concentration processes of 3 were under nitrogen protection.
In a dropwise manner, 8.64 mL (2.5 mol/L, 21.6 mmol) of n-BuLi was added to a solution of 18 mmol of alkynes (4) in THF (16 mL) at − 78 °C, and the mixture was stirred at − 78 °C for 1 h. The solution of 3 in THF was added, and the obtained mixture was gradually warmed to room temperature. After stirring for 16 h, the reaction was quenched with water and extracted with ethyl acetate. The solution of organic phase was removed under a reduced pressure, and the residue was purified by flash column chromatography on silica gel with n-hexane and triethylamine (v/v = 100:1) to give silicon-bridged diynes.
Spectroscopic data
1-[(dimethyl)(phenylethynyl)silyl]-2-(phenylethynyl)benzene ( 5a ) [ 16 ]:
1H NMR (400 MHz, CDCl3): δ 7.82–7.79 (m, 1H), 7.48 (ddd, J = 6.4, 5.2, 2.9 Hz, 3H), 7.40–7.36 (m, 2H), 7.33–7.24 (m, 5H), 7.23–7.15 (m, 3H), 0.57 (s, 6H)
1-[(dimethyl)(pentylethynyl)silyl]-2-(phenylethynyl)benzene ( 5b ) [ 16 ]:
1H NMR (400 MHz, CDCl3): δ 7.87–7.83 (m, 1H), 7.57–7.53 (m, 3H), 7.39–7.32 (m, 5H), 2.27 (t, J = 7.2 Hz, 2H), 1.58–1.49 (m, 3H), 1.42–1.24 (m, 5H), 0.88 (t, J = 7.1 Hz, 3H), 0.56 (s, 6H)
1-[(dimethyl)(phenylethynyl)silyl]-2-(pentylethynyl)benzene ( 5c ):
1H NMR (400 MHz, CDCl3): δ 7.84–7.81 (m, 1H), 7.47 (ddt, J = 7.7, 3.8, 1.9 Hz, 3H), 7.40–7.37 (m, 1H), 7.28–7.25 (m, 4H), 2.38 (t, J = 7.2 Hz, 2H), 1.58 (p, J = 7.3 Hz, 2H), 1.41–1.35 (m, 2H), 1.32–1.26 (m, 2H), 0.86 (t, J = 7.2 Hz, 3H), 0.56 (s, 6H). 13C NMR (101 MHz, CDCl3): δ 138.64, 135.13, 132.35, 132.13, 129.51, 128.70, 128.34, 126.99, 123.33, 107.05, 94.24, 92.72, 81.79, 31.39, 28.35, 22.35, 19.70, 14.08, − 0.60. LC–MS, m/z: 331.3 [M + H]+
General Preparation Procedure for Dibenzosilole Derivatives (6)
First, 2.13 mL of n-BuLi (1.6 mol/L, 3.4 mmol) was added dropwise to a mixture of bis(cyclopentadienyl)zirconium dichloride (Cp2ZrCl2, 0.5 g, 1.7 mmol) and 5 mL of THF at − 78 °C, and the mixture was stirred for 1 h. Then, 5 (1 mmol) was added, and the resulting mixture was heated to 50 °C for 30–40 min (5a: 30 min, 5b–c: 40 min). The mixture was cooled to 0 °C and cuprous chloride (CuCl, 0.38 g, 3 mmol) was added, and the resulting mixture was warmed to room temperature for 10 min. Dimethyl acetylenedicarboxylate or diethyl acetylenedicarboxylate (DMAD or DEAD, 4 mmol) was added and the resulting mixture was heated to 50 °C for 15–90 min (6a: 15 min, 6b: 90 min, 6c–d: 30 min). The reaction was quenched with saturated ammonium chloride solution and extracted with ethyl acetate. The solvent of organic phase was removed under a reduced pressure, and the residue was purified by flash column chromatography on silica gel with n-hexane and ethyl acetate (v/v = 5:1) to give 6.
Spectroscopic data
Dimethyl 9,9-dimethyl-1,4-diphenyl-9-silafluorene-2,3-dicarboxylate ( 6a ) [ 16 ]:
1H NMR (400 MHz, CDCl3): δ 7.53–7.29 (m, 11H), 7.14 (t, J = 7.2 Hz, 1H), 6.98–6.92 (m, 1H), 6.48 (d, J = 8.2 Hz, 1H), 3.48 (s, 3H), 3.45 (s, 3H), 0.06 (s, 6H).
Diethyl 9,9-dimethyl-1,4-diphenyl-9-silafluorene-2,3-dicarboxylate ( 6b ):
1H NMR (400 MHz, CDCl3): δ 7.56–7.32 (m, 11H), 7.15 (t, J = 7.2 Hz, 1H), 7.00–6.92 (m, 1H), 6.49 (d, J = 8.1 Hz, 1H), 4.01–3.90 (m, 4H), 0.96 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H), 0.07 (s, 6H). 13C NMR (101 MHz, CDCl3): δ 168.20, 167.81, 151.95, 147.21, 146.58, 145.79, 143.33, 141.62, 141.01, 139.04, 137.17, 135.04, 129.58, 129.46, 129.17, 128.55, 127.88, 127.59, 127.42, 126.58, 61.20, 61.09, 13.52, 13.37, − 2.51. LC–MS, m/z: 507.1 [M + H]+
Dimethyl 9,9-dimethyl-4-pentyl-1-phenyl-9-silafluorene-2,3-dicarboxylate ( 6c ):
1H NMR (400 MHz, CDCl3): δ 7.59 (d, J = 7.0 Hz, 1H), 7.47–7.40 (m, 3H), 7.35–7.30 (m, 2H), 7.17 (t, J = 7.15 Hz, 1H), 7.00–6.91 (m, 1H), 6.47 (d, J = 8.2 Hz, 1H), 3.88 (s, 3H), 3.44 (s, 3H), 3.03–2.94 (m, 2H), 1.70–1.60 (m, 2H), 1.52–1.36 (m, 4H), 0.96 (t, J = 7.1 Hz, 3H), 0.53 (s, 6H). δ 168.52, 168.38, 147.50, 146.67, 145.86, 142.88, 140.23, 138.95, 137.77, 133.85, 131.88, 129.31, 128.26, 127.75, 127.22, 126.13, 51.95, 51.44, 36.05, 32.15, 22.14, 13.72, − 2.84. LC–MS, m/z: 473.2 [M + H]+
Dimethyl 9,9-dimethyl-1-pentyl-4-phenyl-9-silafluorene-2,3-dicarboxylate ( 6d ):
1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 8.2 Hz, 1H), 7.65 (dd, J = 7.1, 1.5 Hz, 1H), 7.53 (td, J = 7.9, 1.6 Hz, 1H), 7.46–7.41 (m, 3H), 7.39–7.35 (m, 1H), 7.33–7.29 (m, 2H), 3.97 (s, 3H), 3.53 (s, 3H), 3.20–3.14 (m, 2H), 1.93–1.83 (m, 2H), 1.62–1.53 (m, 2H), 1.53–1.45 (m, 2H), 1.02 (t, J = 7.2 Hz, 3H), 0.08 (s, 6H). 13C NMR (101 MHz, CDCl3): δ 169.61, 168.48, 148.19, 147.22, 144.38, 144.01, 142.19, 141.01, 137.00, 135.52, 133.26, 132.55, 132.26, 130.10, 129.23, 127.47, 126.15, 52.36, 51.84, 32.04, 29.47, 22.26, 13.97, − 2.47. LC–MS, m/z: 473.1 [M + H]+
Synthesis of Dimethoxycarbonyl-Substituted Benzonaphthosilole Derivative
Benzonaphthosilole derivative with propyl, phenyl, and dimethoxycarbonyl substituents was synthesized from 4-octyne, phenylacetylene, and 1,4-dibromobenzene, and the synthetic route is shown in Scheme 3.
Preparation Procedure for 1,4-Dibromo-2,5-Diiodobenzene
The preparation of 1,4-dibromo-2,5-diiodobenzene does not need nitrogen protection. First, 60 mL of H2SO4 (98%) was added dropwise to periodic acid (H5IO6, 2.66 g, 11.7 mmol) at 0 °C. Potassium iodide (KI, 5.82 g, 35.1 mmol) was added four times at intervals of 10 min, and 15 min later, 1,4-dibromobenzene (5.52 g, 23.4 mmol) and H2SO4 (98%, 24 mL) were added slowly. The mixture was stirred at 0 °C for 12 h and warmed overnight to room temperature. The obtained mixture was poured in ice water and filtered, and the cake was dissolved in chloroform and washed with NaOH solution (5%). The organic phase solution was removed under reduced pressure, and the residue was recrystallized with chloroform and THF (v:v = 2:1) to give 1,4-dibromo-2,5-diiodobenzene.
Spectroscopic data
1,4-dibromo-2,5-diiodobenzene [ 25 ]:
1H NMR (400 MHz, CDCl3): δ 8.04 (s, 2H).
Preparation Procedure for Benzonaphthosilole Derivative (11)
First, 15.6 mL of n-BuLi (2.5 mol/L, 39 mmol) was added dropwise to the mixture of Cp2ZrCl2 (5.9 g, 19.5 mmol) and THF (100 mL) at − 78 °C, and the resulting mixture was stirred for 1 h. Then, 4-octyne (3.31 g, 30 mmol) was added, and the mixture was stirred at room temperature for 2 h and cooled to 0 °C. Cuprous chloride (4.45 g, 45 mmol) was added, and the mixture was stirred at room temperature for 10 min. Subsequently, 1,3-dimethyl-tetrahydropyrimidin-2(1H)-one (DMPU, 7.3 mL, 60 mmol) and 1,4-dibromo-2,5-diiodobenzene (14.63 g, 30 mmol) were added, and the obtained mixture was heated to 50 °C for 2 h. The reaction was quenched with dilute hydrochloric acid and extracted with ethyl acetate. The organic phase solvent was removed under a reduced pressure and the residue was purified by flash column chromatography on silica gel with n-hexane to give 6-bromo-7-iodo-1,2,3,4-tetrapropylnaphthalene (7).
The synthesis procedures of 6-bromo-7-phenylethynyl-1,2,3,4-tetrapropylnaphthalene (8), 6-chlorodimethylsily-7-phenylethynyl-1,2,3,4-tetrapropylnaphthalene (9), and 6-(phenylethynyl)dimethylsily-7-phenylethynyl-1,2,3,4-tetrapropylnaphthalene (10) are the same as those of 2, 3, and 5.
In a dropwise manner, 2.13 mL of n-BuLi (1.6 mol/L, 3.4 mmol) was added to the mixture of Cp2ZrCl2 (0.5 g, 1.7 mmol) and THF (5 mL) at − 78 °C, and the mixture was stirred for 1 h. Then, 10 (0.56 g, 1 mmol) was added, and the obtained mixture was heated to 70 °C for 1 h. The mixture was cooled to 0 °C, and CuCl (0.38 g, 3 mmol) was added, and then, the mixture was warmed to room temperature for 10 min. Subsequently, DMAD (0.57 g, 4 mmol) was added, and the mixture was heated to 65 °C for 2 h. The reaction was quenched with saturated ammonium chloride solution and extracted with ethyl acetate. The organic phase solvent was removed under a reduced pressure, and the residue was purified by flash column chromatography on silica gel with n-hexane and ethyl acetate (v/v = 5:1) to give dimethyl 5,5-dimethyl-7,10-diphenyl-1,2,3,4-tetrapropylbenzo[b]naphtho[2,3-d]silole-8,9-dicarboxylatepropyl (11).
Spectroscopic data
6-bromo-7-iodo-1,2,3,4-tetrapropylnaphthalene ( 7 ):
1H NMR (400 MHz, CDCl3): δ 8.50 (s, 1H), 8.24 (s, 1H), 2.93 (ddd, J = 11.3, 5.3, 3.0 Hz, 4H), 2.76–2.69 (m, 4H), 1.68–1.55 (m, 8H), 1.15–1.09 (m, 12H)
6-bromo-7-phenylethynyl-1,2,3,4-tetrapropylnaphthalene ( 8 ):
1H NMR (400 MHz, CDCl3): δ 8.19 (d, J = 6.8 Hz, 1H), 7.67–7.63 (m, 1H), 3.01–2.90 (m, 2H), 2.71 (ddd, J = 11.7, 4.9, 3.1 Hz, 2H), 1.71–1.61 (m, 2H), 1.61–1.54 (m, 2H), 1.15–1.07 (m, 6H)
6-(phenylethynyl)dimethylsily-7-phenylethynyl-1,2,3,4-tetrapropylnaphthalene ( 10 ):
1H NMR (400 MHz, CDCl3): δ 8.64 (s, 1H), 8.22 (s, 1H), 7.66–7.63 (m, 2H), 7.55–7.52 (m, 2H), 7.37 (q, J = 2.5 Hz, 3H), 7.33–7.30 (m, 3H), 3.04 (dd, J = 7.3, 4.4 Hz, 4H), 2.75 (dd, J = 7.9, 4.5 Hz, 4H), 1.74–1.69 (m, 4H), 1.59 (dt, J = 6.7, 2.1 Hz, 4H), 1.14 (ddd, J = 13.0, 7.4, 2.8 Hz, 12H), 0.73 (s, 6H)
Dimethyl 5,5-dimethyl-7,10-diphenyl-1,2,3,4-tetrapropylbenzo[b]naphtho[2,3-d]silole-8,9-dicarboxylatepropyl ( 11 ):
1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H), 7.66 (s, 1H), 7.50 (dt, J = 4.9, 2.5 Hz, 3H), 7.46–7.40 (m, 5H), 7.34 (dd, J = 7.3, 2.1 Hz, 2H), 3.47 (s, 3H), 3.43 (s, 3H), 2.96 (dd, J = 9.4, 6.6 Hz, 2H), 2.68–2.62 (m, 2H), 2.61–2.55 (m, 2H), 2.23 (t, J = 8.0 Hz, 2H), 1.62 (q, J = 7.8 Hz, 2H), 1.48 (ddt, J = 16.3, 12.0, 7.9 Hz, 6H), 1.16 (q, J = 7.6 Hz, 2H), 1.09–1.00 (m, 10H), 0.11 (s, 6H). 13C NMR (101 MHz, CDCl3): δ 168.30, 147.81, 146.10, 141.45, 141.07, 139.60, 138.05, 137.58, 136.73, 135.62, 135.10, 133.83, 131.72, 130.17, 129.32, 128.01, 127.68, 51.97, 32.57, 24.58, 14.77, − 1.58. LC–MS, m/z: 697.2 [M + H]+
Results and Discussion
In this study, new zirconium-mediated cycloaddition strategies for synthesizing several novel dibenzosilole and benzonaphthosilole derivatives are developed. Using these methods, EWG, alkyl, and phenyl groups can be selectively introduced onto the aromatic rings of these new derivatives.
The Sonogashira coupling of 1 and 1-bromo-2-iodobenzene in the presence of Pd(PPh3)2Cl2 and CuI produced high yields of 2 and 8. However, when Pd(Ph3)4 and CuI were used as catalysts, the reaction was sluggish and did not accelerate with an increase in temperature. This suggests that Pd(PPh3)2Cl2 is the suitable catalyst for this reaction. Since iodine is more reactive than bromine, the Sonogashira coupling occurred selectively at the iodine position, and the introduction of R1 and R2 groups could be effectively controlled.
Side reactions occurred when Me2SiCl2 was added dropwise during the synthesis of 3 and 9. These reactions reduced when Me2SiCl2 was added in one portion. This indicates that insufficient amount of Me2SiCl2 would react with double its amount of lithiated molecules of 2 and 8 to give by-products, and adding Me2SiCl2 in one portion reduced the by-products. To prevent overheating in the process of adding Me2SiCl2 in one portion, Me2SiCl2 and the reaction mixture were first cooled to − 78 °C. Given that Si–Cl bonds in 3 and 9 can hydrolyze easily, n-hexane was used as the quencher. In addition, the corresponding quenching, filtration, and concentration operations were carried out under nitrogen atmosphere.
The reaction mechanism of zirconium-mediated cycloaddition of silicon-bridged diynes is shown in Scheme 4. It has been reported that zirconacyclopentadienes can react with electron-withdrawing alkynes to give benzene derivatives [26, 27]. It was speculated that silolozirconacyclopentadiene intermediates were formed from the reaction of silicon-bridged diynes with Cp2ZrBu2. The treatment of silolozirconacyclopentadienes with CuCl generated copper-substituted butadiene intermediates, which was followed by an insertion reaction with EWG-substituted alkynes at the C–Cu bond, and copper-substituted 1,3,5-hexatriene intermediates were produced. Then a six-membered ring molecule was formed via Michael addition reaction. Afterward, Cu(I) at C–Cu bond was reduced to Cu(0), at which point EWG-substituted silole derivatives were formed. Through this reaction, Cu(I) was converted to Cu(0), which suggests that CuCl was not a catalyst but a reactant. Moreover, a copper mirror was observed on the inner wall of the bottle after this reaction.
As for the formation of silolozirconacyclopentadienes of 5, the conversion rate of silicon-bridged diynes was greatly affected by the temperature. As detected by TLC, the conversion rate was low after 3 h of reaction at room temperature. Surprisingly, 30–40 min of stirring at 50 °C resulted in a high conversion rate. The results showed that 50 °C was the optimum temperature for the silolozirconacyclopentadienes formation. In addition, a longer time was needed for this reaction when there was an alkyl group on the silicon-bridged diynes.
To optimize the reaction temperature of the EWG-substituted alkynes cycloaddition, the reaction of 6a was investigated, and different temperatures were tried (25, 35, 50, 60, and 70 °C). It took these reactions 6 h at 25 °C, 1 h at 35 °C, and 15 min at 50 °C to prepare 6a, and by-products were detected by TLC when the temperature was higher than 60 °C. Based on these, 50 °C was chosen as the optimum temperature for the cycloaddition reaction of EWG-substituted alkynes.
The yields of the substances obtained during the preparation of dibenzosilole derivatives are listed in Table 1. Phenyl-, pentyl-, and alkoxycarbonyl-substituted dibenzosilole derivatives were obtained in yields of 64–79% by zirconium-mediated cycloaddition (entries 6–9). In contrast to iridium(I)-catalyzed cycloaddition reaction [16], novel dibenzosilole derivatives were efficiently prepared (entries 7–9), in addition to the high yield of dimethoxycarbonyl-substituted derivative obtained (entry 6). This indicates that zirconium-mediated cycloaddition has good adaptability to silicon-bridged diynes bearing different substituents.
During the synthesis of 11, TLC detected that the silolozirconacyclopentadiene intermediate formation was sluggish at 50 °C. Considering the steric effect of the naphthyl group, 55, 60, 65, and 70 °C were attempted. It took 1 h for the reaction to convert silicon-bridged diynes into intermediates even at 70 °C, and the conversion was slower for lower temperatures. Therefore, 70 °C was selected as the optimum temperature for the formation of the silolozirconacyclopentadiene intermediate of 10.
For the cycloaddition of dimethyl but-2-ynedioate to obtain 11, 50, 55, 60, 65, and 70 °C were tried. The reactions were slow when the temperatures were below 65 °C, and at 70 °C, the separation of 11 was greatly affected by the large amount of by-products. In contrast, 2 h of reaction at 65 °C moderately yielded 11; hence, it was chosen as the optimum temperature for the cycloaddition to prepare 11.
The yields obtained during the preparation of benzonaphthosilole derivative are listed in Scheme 5. As noted above, there are few reports on the synthesis of benzonaphthosilole derivatives. Our report on the synthesis of 11 is unique, because to date, it is the only synthesized benzonaphthosilole derivative with substituents on the aromatic rings. The structure and synthesis strategy of 11 are rather different from those of the previous literature [17,18,19]. In contrast to the rhodium-catalyzed reaction, the synthesis strategy we designed uses readily available alkynes as the starting materials instead of dimethyl[2-(naphthalen-2-yl)phenyl]silane, which is difficult to prepare. Moreover, propyl, phenyl and dimethoxycarbonyl groups can be selectively introduced onto the aromatic rings of 11 by combining zirconium-mediated coupling reaction, Sonogashira coupling reaction, and the novel zirconium-mediated cycloaddition reported in this paper. The result of preparing 11 shows that zirconium-mediated cycloaddition has excellent adaptability to silicon-bridged diynes bearing a larger group as naphthyl. This indicates that zirconium-mediated cycloaddition reactions of silicon-bridged diynes can be used to synthesize various silole derivatives.
Conclusions
In summary, a new method of synthesizing alkoxycarbonyl-substituted dibenzosilole derivatives was developed, and the preparation of 5c, 6b, 6c, and 6d was reported for the first time. High yields of dibenzosilole derivatives were obtained, which were much higher than that of iridium(I)-catalyzed cycloaddition. In addition, we successfully designed a strategy based on zirconium-mediated cycloaddition reaction for synthesizing a moderate yield of benzonaphthosilole derivative bearing several substituents, which suggests that this reaction is suitable to silicon-bridged diynes bearing different groups. By synthesizing compound 11, we successfully accomplished the selective and multiple derivatization of benzonaphthosilole. Using the synthesis strategies we reported, alkoxycarbonyl, phenyl, and alkyl-substituted dibenzosilole and benzonaphthosilole derivatives were efficiently synthesized, where the alkoxycarbonyl, phenyl, and alkyl groups were selectively introduced onto the aromatic rings. All raw materials of the cycloaddition reactions are available or easily prepared. In addition, alkoxycarbonyl groups have potential for further derivatization, which is of great significance for expanding the range of applications of silole derivatives. These novel zirconium-mediated cycloaddition synthesis strategies can provide a wider variety of silole derivatives.
References
Hong YN, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388
Tamao K, Uchida M, Izumizawa T et al (1996) Silole derivatives as efficient electron transporting materials. J Am Chem Soc 118:11974–11975
Quan CY, Nie H, Hu RR et al (2015) A silole-based efficient electroluminescent material with good electron-transporting potential. Chin J Chem 33:842–846
Anthony JE, Facchetti A, Heeney M et al (2010) N-type organic semiconductors in organic electronics. Adv Mater 22:3876–3892
Erlik O, Unlu NA, Hizalan G et al (2015) Silafluorene-based polymers for electrochromic and polymer solar cell applications. J Polym Sci Pol Chem 53:1541–1547
Duan CH, Cai WZ, Huang F et al (2010) Novel silafluorene-based conjugated polymers with pendant acceptor groups for high performance solar cells. Macromolecules 43:5262–5268
Yuan MJ, Yang PY, Durban MM et al (2012) Low bandgap polymers based on silafluorene containing multifused heptacylic arenes for photovoltaic applications. Macromolecules 45:5934–5940
Wang E, Li C, Zhuang WL et al (2008) High-efficiency red and green light-emitting polymers based on a novel wide bandgap poly(2,7-silafluorene). J Mater Chem 18:797–801
Yang J, Aschemeyer S, Martinez HP et al (2010) Hollow silica nanospheres containing a silafluorene-fluorene conjugated polymer for aqueous TNT and RDX detection. Chem Commun 46:6804–6806
Ouyang KB, Liang Y, Xi ZF (2012) Construction of benzosiloles, six- and eight-membered silacyclic skeletons, via a Pd-catalyzed intramolecular Mizoroki–Heck Reaction of vinylsilanes. Org Lett 14(17):4572–4575
Li LC, Xiang JF, Xu CH (2007) Synthesis of novel ladder bis-silicon-bridged p-terphenyls. Org Lett 9(23):4877–4879
Murai M, Matsumoto K, Okada R et al (2014) Rhodium-catalyzed dehydrogenative germylation of C-H bonds: new entry to unsymmetrically functionalized 9-germafluorenes. Org Lett 16:6492–6495
Xu L, Zhang S, Li PF (2015) Synthesis of silafluorenes and silaindenes via silyl radicals from arylhydrosilanes: intramolecular cyclization and intermolecular annulations with alkynes. Org Chem Front 2:459–463
Murata M, Takizawa M, Sasaki H et al (2016) Synthesis of dibenzosiloles via Platinum-catalyzed intramolecular dehydrogenative cyclization of 2-(Dialkylsilyl)biaryls. Chem Lett 45:857–859
Hudrlik PF, Dai DH, Hudrlik AM (2006) Reactions of dilithiobutadienes with monochlorosilanes: observation of facile loss of organic groups from silicon. J Organomet Chem 691:1257–1264
Matsuda T, Kadowaki S, Goya T et al (2007) Synthesis of silafluorenes by Iridium-catalyzed [2 + 2+2] cycloaddition of silicon-bridged diynes with alkynes. Org Lett 9(1):133–136
Murai M, Okada R, Asako S et al (2017) Rhodium-catalyzed silylative and germylative cyclization with dehydrogenation leading to 9-sila- and 9-germafluorenes: a combined experimental and computational mechanistic study. Chem Eur J 23:10861–10870
Ureshino T, Yoshida T, Kuninobu Y et al (2010) Rhodium-catalyzed synthesis of silafluorene derivatives via cleavage of silicon–hydrogen and carbon–hydrogen bonds. J Am Chem Soc 132:14324–14326
Murai M, Okada R, Nishiyama A et al (2016) Synthesis and properties of sila[n]helicenes via dehydrogenative silylation of C–H bonds under Rhodium catalysis. Org Lett 18:4380–4383
Seri T, Qu HM, Zhou LS et al (2008) Substituent effects in the preparation of naphthacenes by the coupling reaction of diyne-derived zirconacyclopentadienes with tetraiodobenzene. Chem Asian J 3:388–392
Li S, Qu HM, Zhou LS et al (2009) Zirconium-mediated selective synthesis of 1,2,4,5-tetrasubstituted benzenes from two silyl-substituted alkynes and one internal alkyne. Org Lett 11(15):3318–3321
Wang H, Li JQ, Zhou LS et al (2015) Palladium-catalyzed synthesis of 1,2,3,4-tetraalkyl-1,4-diarylbutadienes by cross-coupling of zirconacyclopentadienes with aryl iodides. Chin Chem Lett 26:1303–1306
Arndt S, Hansmann MM, Motloch P et al (2017) Intramolecular anti-phosphinoauration of alkynes: an FLP-motivated approach to stable aerated phosphindolium complexes. Chem Eur J 23:2542–2547
Just G, Singh R (1987) The synthesis of 11-13-membered diacetylenic and 18-membered tetraacetylenic ring systems. Tetrahedron Lett 28(48):5981–5984
Duhovic S, Dinca M (2015) Synthesis and electrical properties of covalent organic frameworks with heavy chalcogens. Chem Mater 27:5487–5490
Takahashi T, Xi ZF, Yamazaki A et al (1998) Cycloaddition reaction of zirconacyclopentadienes to alkynes: highly selective formation of benzene derivatives from three different alkynes. J Am Chem Soc 120:1672–1680
Li JQ, Zhang JQ, Qu HM et al (2016) Zirconium-mediated selective synthesis of 1,4-dialkyl(aryl)-hexa-substituted benzenes from two silyl-substituted alkynes and one internal alkyne. Chem Res Chin Univ 32(3):366–372
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 21102099).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Qu, H., Zhang, X., Chen, X. et al. Zirconium-Mediated Synthesis of Multi-substituted Dibenzosilole and Benzonaphthosilole Derivatives. Trans. Tianjin Univ. 24, 538–546 (2018). https://doi.org/10.1007/s12209-018-0154-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-018-0154-6