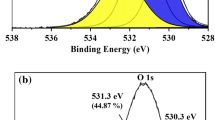
Abstract
In this study, liquid-phase aerobic oxidation of toluene catalyzed by Mn–Mo oxide was conducted in a 1.0 L batch reactor. The macroscopic kinetics of toluene consumption and benzaldehyde generation at 413–443 K were obtained from a combination of experimental observation and hypothetical models. The results clearly showed that both the oxidation rate of toluene and generation rate of the aromatic product were proportional to the concentration of the substrate, the partial pressure of oxygen and the surface area of the catalyst. The energy barrier of toluene oxidation to benzyl alcohol was the highest (≈ 81 kJ mol−1), while that of benzyl alcohol oxidation to benzaldehyde was the lowest (≈ 57 kJ mol−1). Moreover, the activation energy of further oxidation of benzaldehyde in an acetic acid solvent was only slightly lower (≈ 1.9 kJ mol−1) than that of toluene oxidation. Significantly, the transformation of benzyl alcohol indeed contributed to the generation of benzaldehyde and this step conformed to a first-order parallel-consecutive model. Increased reaction temperature and residence time favored the transformation of benzyl alcohol to benzaldehyde. In addition, doping with molybdenum at Mn/Mo = 3/1 enhanced the catalytic performance of the heterogeneous catalyst and was attributed to the presence of a synergetic effect between different metal cations. Regarding the microscopic kinetics, the LH-OS-ND mechanism (Langmuir–Hinshelwood adsorption of reagents on the same type of active sites and non-dissociative adsorption of oxygen) was verified as responsible for the heterogeneous oxidation of toluene. Oxygen and benzaldehyde were weakly adsorbed (ΔHads,Oxy ≈ − 15 kJ mol−1, ΔHads,Bald ≈ − 30 kJ mol−1), but showed strong mobility (ΔSads,Oxy ≈ − 22 J mol−1 K−1), ΔSads,Bald ≈ − 39 J mol−1 K−1). The fundamental intrinsic rates were deduced based on the LH-OS-ND mechanism and showed great consistency with the macroscopic results.









Similar content being viewed by others
References
Debono O, Thevenet F, Gravejat P et al (2011) Toluene photocatalytic oxidation at ppbv levels: kinetic investigation and carbon balance determination. Appl Catal B 106(3–4):600–608
Sleiman M, Conchon P, Ferronato C et al (2009) Photocatalytic oxidation of toluene at indoor air levels (ppbv): towards a better assessment of conversion, reaction intermediates and mineralization. Appl Catal B 86(3–4):159–165
Konietzni F, Kolb U, Dingerdissen U et al (1998) AMM-MnxSi-catalyzed selective oxidation of toluene. J Catal 176(2):527–535
Wang JQ, Fang H, Li Y et al (2006) Liquid phase oxidation of p-chlorotoluene to p-chlorobenzaldehyde over cobalt-doped mesoporous titania with a crystalline framework. J Mol Catal A 250(1):75–79
Lv JG, Shen Y, Peng LM et al (2010) Exclusively selective oxidation of toluene to benzaldehyde on ceria nanocubes by molecular oxygen. Chem Comm 46(32):5909–5911
Acharyya SS, Ghosh S, Tiwari R et al (2014) Preparation of the CuCr2O4 spinel nanoparticles catalyst for selective oxidation of toluene to benzaldehyde. Green Chem 16(5):2500–2508
Wu XK, Deng ZL, Yan JJ et al (2014) Effect of acetic anhydride on the oxidation of toluene to benzaldehyde with metal/bromide catalysts. Ind Eng Chem Res 53(38):14601–14606
Bastos SST, Carabinerio SAC, Orfao JJM et al (2012) Total oxidation of ethyl acetate, ethanol and toluene catalyzed by exotemplated manganese and cerium oxides loaded with gold. Catal Today 180(1):148–154
Santos VP, Bastos SST, Pereira MFR et al (2010) Stability of a cryptomelane catalyst in the oxidation of toluene. Catal Today 154(3):308–311
Czytko MP, Bub GK (1981) Oxidation of toluene by cobalt (III) acetate in acetic acid solution: influence of water. Ind Eng Chem Res 20(3):481–486
Papadatos K, Shelstad KA (1973) Catalyst screening using a stone DTA Apparatus: I. Oxidation of toluene over cobalt-metal-oxide catalysts. J Catal 28(1):116–123
Morimoto T, Ogata Y (1967) Kinetics of the autoxidation of toluene catalyzed by cobaltic acetate. J Chem Soc B 12:62–66
Helfferich FG (2001) Kinetics of homogeneous multistep reactions. In: Compton RG, Hancock G (eds) Comprehensive. Chemical kinetics, vol 38. Elsevier, Amsterdam, pp 283–286
Bhattacharya D, Guha DK, Roy AN (1973) Liquid phase air oxidation of toluene to benzoic acid. II. Kinetics and mechanism. Chem Age India 24:87–90
Hoorn JAA, Soolingen JV, Verseeg GF (2005) Modelling toluene oxidation: incorporation of mass transfer phenomena. Chem Eng Res Des 83(2):187–195
Liao YN, Fu ML, Chen LM et al (2013) Catalytic oxidation of toluene over nanorod-structured Mn–Ce mixed oxides. Catal Today 216(1):220–228
Kesavan L, Tiruvalam R, Rahim MHA et al (2011) Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au–Pd alloy nanoparticles. Science 331(6014):195–199
Petrik LS, Krylova GV, Kelyp OO et al (2015) XPS and TPR study of sol-gel derived M/TiO2 powders (M=Co, Cu, Mn, Ni). Chem Phys Technol Surf 6(2):179–189
Worayingyong A, Kangvansura P, Ausadasuk S et al (2008) The effect of preparation: pechini and Schiff base methods on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts. Colloids Surf A 315(1–3):217–225
Chen DK, He DD, Lu JC et al (2017) Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl Catal B 218:249–259
Ma JJ, Yu J, Chen WQ et al (2016) The effect of water on the oxidation of toluene catalyzed by manganese complex oxide. Catal Lett 146(8):1600–1610
Gizli A, Aytimur G, Alpay E et al (2010) Catalytic liquid phase oxidation of toluene to benzoic acid. Chem Eng Technol 31(3):409–416
Tang SW, Liang B (2007) Kinetics of the liquid-phase oxidation of toluene by air. Ind Eng Chem Res 46(20):6442–6448
Barbaro A, Larrondo S, Amadeo N (2000) Oxidation of toluene to benzaldehyde over VSb1−xTixO4 catalyst kinetics studies. Stud Surf Sci Catal 130:1733–1738
Bawn CEH, Jolley JE (1956) The cobalt salt catalyzed autoxidation of benzaldehyde. Proc R Soc A 237(1210):297–312
Ingles TA, Melville HW (1953) The kinetics of the oxidation of mixtures of benzaldehyde and n-decana. Proc R Soc A 218(1133):163–175
Komissarova IN, Komissarov VD, Denisov ET (1978) The mechanism of benzaldehyde oxidation by ozonated oxygen. Russ Chem Bull 27(9):1751–1756
Banerji KK (1968) Kinetics and mechanism of the oxidation of substituted benzaldehydes by N-Bromobenzamide. J Org Chem 51(25):4764–4767
Partenheimer W (2006) The high yield synthesis of benzaldehydes from benzylic alcohols using homogeneously catalyzed aerobic oxidation in acetic acid. Adv Synth Catal 348(4–5):559–568
Zhan GW, Hong YL, Lu FF et al (2013) Kinetics of liquid phase oxidation of benzyl alcohol with hydrogen peroxide over bio-reduced Au/TS-1 catalysts. J Mol Catal A 366(1):215–221
Behera GC, Parida KM (2012) Liquid phase catalytic oxidation of benzyl alcohol to benzaldehyde over Vanadium phosphate catalyst. Appl Catal A 413–414:245–253
Saeed M, Ilyas M, Siddique M (2015) Kinetics of lab prepared manganese oxide catalyzed oxidation of benzyl alcohol in the liquid phase. Int J Chem Kinet 47(7):447–460
Savara A, Rossetti I, Chan-Thaw CE et al (2016) Microkinetic modeling of benzyl alcohol oxidation on carbon-supported palladium nanoparticles. Chem Cat Chem 8(15):2482–2491
Jin SJ, Chen JZ (2014) Preparation of benzaldehyde by aerobic liquid-phase oxidation of toluene with Co (acac)2. J Chem Eng Chin Univ 28(2):311–316
Tan PH, Tang SW, Liang B (2010) Kinetic models for liquid-phase catalytic oxidation of toluene to benzoic acid with pure oxygen. Chem Eng Comm 197(7):953–962
Zhang GQ, Zhang X, Lin T et al (2012) Synergetic effect of FeVO4 and α-Fe2O3 in Fe–V–O catalysts for liquid phase oxidation of toluene to benzaldehyde. Chin Chem Lett 23(2):145–148
Li J, Fisher CL, Chen JL et al (1996) Calculation of redox potentials and pKa values of hydrated transition metal cations by a combined density functional and continuum dielectric theory. Inorg Chem 35(16):4694–4702
Border-Richard E, Vedrine J (2014) ChemInform abstract: selective redox catalysis. ChemInform 45(16):1–17
Tarjomannejad A, Farzi A, Niaei A et al (2016) An experimental and kinetic study of toluene oxidation over LaMn1−xBxO3 and La0.8A0.2Mn0.3B0.7O3 (A = Sr, Ce and B = Cu, Fe) nano-perovskite catalysts. Korean J Chem Eng 33(9):2628–2637
Ilyas M, Khan H (2007) Kinetics of the surface catalyzed reactions: application of power rate law. React Kinet Catal Lett 92(1):75–82
Saeed M, Ilyas M (2013) Oxidative removal of phenol from water catalyzed by nickel hydroxide. Appl Catal B 129(1):247–254
Saeed M, Ilyas M, Siddique M (2013) Oxidative degradation of oxalic acid in aqueous medium using manganese oxide as catalyst at ambient temperature and pressure. Arab J Sci Eng 38(7):1739–1748
Saeed M, Ilyas M, Siddique M (2012) Oxidative degradation of phenol in aqueous medium catalyzed by lab prepared cobalt oxide. J Chem Soc Pak 34(3):626–633
Smeds S, Murzin D, Salmi T (1995) Kinetics of ethyl benzene hydrogenation on Ni/Al2O3. Appl Cata A 125(2):271–291
Antonello A, Barresi GB (1994) Deep catalytic oxidation of aromatic hydrocarbon mixtures: reciprocal inhibition effects and kinetics. Ind Eng Chem Res 33(12):2964–2974
Savara A, Chan-Thaw CE, Rossetti I et al (2014) Benzyl alcohol oxidation on carbon-supported Pd nanoparticles: elucidating the reaction mechanism. Chem Cat Chem 6(12):3464–3473
Jin SS, Zhang LM, He JZ (2008) Review of reactions of manganese oxides with organic compounds and applications of MnOx in environmental remediation. Acta Sci Circum 28(12):2394–2401
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 21376163).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Zhang, Q. & Zeng, A. Kinetics and Mechanism Modeling of Liquid-Phase Toluene Oxidation to Benzaldehyde Catalyzed by Mn–Mo Oxide. Trans. Tianjin Univ. 25, 52–65 (2019). https://doi.org/10.1007/s12209-018-0146-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-018-0146-6