Abstract
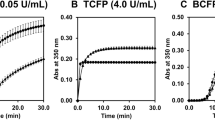
We identified two heterozygous dysfibrinogenemias, Bβp.Gly45Cys (Kyoto VII; K-VII) and Bβp.Arg74Cys (Iida II; I-II). The impairment of polymerization of Bβp.G45C has been well analyzed; however, that of Bβp.R74C has not. Thus, we compared fibrin polymerization between these variants. To determine the structural and functional characterization of purified fibrinogens, we performed immunoblotting analysis, kinetic analyses of fibrinopeptide A and B release, and thrombin- or batroxobin-catalyzed fibrin or fibrin monomer polymerization. Immunoblotting analysis showed that both variant fibrinogens had variant fibrinogen-albumin complexes and variant fibrinogen multimers, and the amounts of fibrinogen-albumin complexes with fibrinogen K-VII was more than with fibrinogen I-II. Moreover, fibrinopeptide B release from fibrinogen K-VII was about 50% of the control, whereas the others were normal. The maximum slopes of polymerization for variant fibrinogens were reduced, but fibrinogen K-VII was reduced more than fibrinogen I-II. The present study demonstrated that both Bβp.G45C and Bβp.R74C variants showed the presence of variant fibrinogen-albumin complexes and variant fibrinogen multimers, and polymerization of Bβp.G45C was impaired more than Bβp.R74C. Our study and several previous reports concerning the clinical phenotype of both variants suggested the risks of bleeding for patients with Bβp.G45C and thrombosis for patients with Bβp.R74C.




Similar content being viewed by others
References
Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–99.
Huang S, Cao Z, Chung DW, Davie EW. The role of betagamma and alphagamma complexes in the assembly of human fibrinogen. J Biol Chem. 1996;271:27942–7.
Medved L, Weisel JW. Haemostasis F and FXS of SSC of IS on T and. Recommendations for nomenclature on fibrinogen and fibrin. J Thromb Haemost. 2009;7:355–9.
Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem. 2017;82:405–56.
Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229.
Mosesson MW, Siebenlist KR, DiOrio JP, Matsuda M, Hainfeld JF, Wall JS. The role of fibrinogen D domain intermolecular association sites in the polymerization of fibrin and fibrinogen Tokyo II (gamma 275 Arg–>Cys). J Clin Invest. 1995;96:1053–8.
Yang Z, Mochalkin I, Doolittle RF. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc Natl Acad Sci USA. 2000;97:14156–61.
Mosesson MW, DiOrio JP, Siebenlist KR, Wall JS, Hainfeld JF. Evidence for a second type of fibril branch point in fibrin polymer networks, the trimolecular junction. Blood. 1993;82:1517–21.
Groupe d’etude sur l’hemostase et la thrombose. Human fibrinogen database. 2018. https://site.geht.org/base-de-donnees-fibrinogene/ cited 2020 Jan 14.
Hirota-Kawadobora M, Terasawa F, Yonekawa O, Sahara N, Shimizu E, Okumura N, et al. Fibrinogens Kosai and Ogasa: Bbeta15Gly–>Cys (GGT–>TGT) substitution associated with impairment of fibrinopeptide B release and lateral aggregation. J Thromb Haemost. 2003;1:275–83.
Koopman J, Haverkate F, Grimbergen J, Engesser L, Nováková I, Kerst AF, et al. Abnormal fibrinogens IJmuiden (B beta Arg14––Cys) and Nijmegen (B beta Arg44––Cys) form disulfide-linked fibrinogen-albumin complexes. Proc Natl Acad Sci USA. 1992;89:3478–82.
Ikeda M, Kobayashi T, Arai S, Mukai S, Takezawa Y, Terasawa F, et al. Recombinant γT305A fibrinogen indicates severely impaired fibrin polymerization due to the aberrant function of hole “A” and calcium binding sites. Thromb Res. 2014;134:518–25.
Mihalyi E. Physicochemical studies of bovine fibrinogen. IV. Ultraviolet absorption and its relation to the structure of the molecule. Biochemistry. 1968;7:208–23.
Okumura N, Terasawa F, Tanaka H, Hirota M, Ota H, Kitano K, et al. Analysis of fibrinogen gamma-chain truncations shows the C-terminus, particularly gammaIle387, is essential for assembly and secretion of this multichain protein. Blood. 2002;99:3654–60.
Soya K, Terasawa F, Okumura N. Fibrinopeptide A release is necessary for effective B: b interactions in polymerisation of variant fibrinogens with impaired A: a interactions. Thromb Haemost. 2013;109:221–8.
Okumura N, Gorkun OV, Lord ST. Severely impaired polymerization of recombinant fibrinogen gamma-364 Asp –> His, the substitution discovered in a heterozygous individual. J Biol Chem. 1997;272:29596–601.
Hirota-Kawadobora M, Kani S, Terasawa F, Fujihara N, Yamauchi K, Tozuka M, et al. Functional analysis of recombinant Bbeta15C and Bbeta15A fibrinogens demonstrates that Bbeta15G residue plays important roles in FPB release and in lateral aggregation of protofibrils. J Thromb Haemost. 2005;3:983–90.
Holm B, Godal HC. Quantitation of the three normally-occurring plasma fibrinogens in health and during so-called “acute phase” by SDS electrophoresis of fibrin obtained from EDTA-plasma. Thromb Res. 1984;35:279–90.
Holm B, Nilsen DW, Kierulf P, Godal HC. Purification and characterization of 3 fibrinogens with different molecular weights obtained from normal human plasma. Thromb Res. 1985;37:165–76.
Kamura T, Tsuda H, Yae Y, Hattori S, Ohga S, Shibata Y, et al. An abnormal fibrinogen Fukuoka II (Gly-B beta 15–>Cys) characterized by defective fibrin lateral association and mixed disulfide formation. J Biol Chem. 1995;270:29392–9.
Kamijyo Y, Hirota-Kawadobora M, Yamauchi K, Terasawa F, Honda T, Ikeya M, et al. Analysis of plasmin generation and clot lysis of plasma fibrinogen purified from a heterozygous dysfibrinogenemia, BbetaGly15Cys (Hamamatsu II). Blood Coagul Fibrinolysis. 2009;20:726–32.
Yoshida N, Wada H, Morita K, Hirata H, Matsuda M, Yamazumi K, et al. A new congenital abnormal fibrinogen Ise characterized by the replacement of B beta glycine-15 by cysteine. Blood. 1991;77:1958–63.
Kibe T, Ikeya M, Yokochi K, Okumura N. Lateral medullary syndrome in a boy with hereditary dysfibrinogenemia. Brain Dev. 2012;34:857–60.
Würtinger P, Griesmacher A, Ivaškevičius V, Biswas A, Zehetbauer S, Oldenburg J, et al. Novel point mutation in fibrinogen (Innsbruck; BβArg44Gly). Phenotypic differences compared to another mutation (fibrinogen Nijmegen) at the same position. Hamostaseologie. 2015;35(Suppl 1):S22–S26.
Shlebak AA, Katsarou AD, Adams G, Fernando F. A novel mutation in exon 2 of FGB caused by c.221G>T substitution, predicting the replacement of the native arginine at position 74 with a Leucine (p.Arg74Leu) in a proband from a Kurdish family with dysfibrinogenaemia and familial venous and art. J Thromb Thrombolysis. 2017;43:263–70.
Engesser L, Koopman J, de Munk G, Haverkate F, Nováková I, Verheijen JH, et al. Fibrinogen nijmegen: congenital dysfibrinogenemia associated with impaired t-PA mediated plasminogen activation and decreased binding of t-PA. Thromb Haemost. 1988;60:113–20.
Marchi R, Rojas H, Meyer M, Castillo O, De Sáez RA, Weisel JW. A novel missense mutation in the FGB g. 3354 T>A (p. Y41N), fibrinogen Caracas VIII. Thromb Haemost. 2011;105:627–34.
Vakalopoulou S, Mille-Baker B, Mumford A, Manning R, Laffan M. Fibrinogen Bbeta14 Arg–>Cys: further evidence for a role in thrombosis. Blood Coagul fibrinolysis an Int J Haemost Thromb. 1999;10:403–8.
Acknowledgements
We gratefully acknowledge Dr. Toru Inaba (Kyoto Prefectural University of Medicine, Kyoto) and Dr. Yu Furui (Shinshu University of School of Medicine, Matsumoto) for the patient referrals. This work was supported by JSPS KAKENHI Grant Number JP17K09009 (Chiaki Taira and Nobuo Okumura).
Author information
Authors and Affiliations
Contributions
TK and MY performed the research, analyzed the data. TK wrote the manuscript. TK, CT, YH, and NO designed the research and discussed the data. NO and TK reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12185_2020_2919_MOESM1_ESM.tif
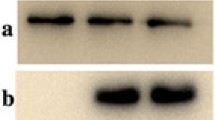
Supplementary file1 (TIF 1194 kb) Supplemental Figure 1: Western blotting analysis under reducing conditions using an anti-human albumin antibody and an anti-human fibrinogen antibody. Purified plasma fibrinogens were analyzed by western blotting in reducing conditions (Panel A: anti-human albumin antibody, Panel B: anti-human fibrinogen antibody). Panel A: the band that reacted with anti-human albumin antibody was shown in both variant fibrinogens. Panel B: these bands showed the typical pattern. Above the dashed line: stacking gel, below the dashed line: resolving gel. 1: normal fibrinogen, 2: fibrinogen Kyoto VII (Bβp.G45C), 3: fibrinogen Iida II (Bβp.R74C). Alb: albumin.
About this article
Cite this article
Kaido, T., Yoda, M., Kamijo, T. et al. Comparison of molecular structure and fibrin polymerization between two Bβ-chain N-terminal region fibrinogen variants, Bβp.G45C and Bβp.R74C. Int J Hematol 112, 331–340 (2020). https://doi.org/10.1007/s12185-020-02919-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02919-5