Abstract
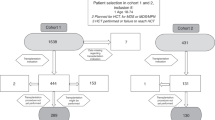
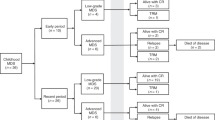
We analyzed the outcomes of allogeneic stem cell transplantation (SCT) and risk factors for chimerism in 108 patients with Wiskott–Aldrich syndrome (WAS) who were registered with The Japan Society for Hematopoietic Cell Transplantation between January 1985 and December 2016. A preparative conditioning regimen consisting of myeloablative conditioning (MAC) was provided to 76 patients, and reduced-intensity conditioning was provided to 30 patients. Fifty-one patients received prophylaxis against graft-versus-host disease (GVHD) with cyclosporine, and 51 patients received tacrolimus (Tac). Chimerism analyses had been performed in 91 patients. Neutrophil engraftment was achieved in 91 patients (84.3%). The engraftment rate was significantly higher in patients who received Tac for GVHD prophylaxis (p = 0.028). Overall survival rate (OS) was significantly higher in patients with complete chimerism than in patients with mixed chimerism (88.2 ± 6.1% and 66.7 ± 9.9%, respectively, p = 0.003). Multivariate analysis showed that the rate of complete chimerism in patients who received MAC including cyclophosphamide (CY) at a dose of 200 mg/kg was significantly higher (p = 0.021) than that in patients who received other conditioning. Thus, MAC including CY at a dose of 200 mg/kg and Tac for GVHD prophylaxis were optimal conditions of SCT for patients with WAS under existing study.

Similar content being viewed by others
References
Familiärer WA. angeborener Morbus Werlhofii. Monatsschr Kinderheilkd. 1937;68:212–6 (in German).
Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1937;13:133–9.
Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell. 1994;78:635–44.
Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott–Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980–2009: an international collaborative study. Blood. 2011;118:1675–84.
Kobayashi R, Ariga T, Nonoyama S, Kanegane H, Tsuchiya S, Morio T, et al. Outcome in patients with Wiskott–Aldrich syndrome following stem cell transplantation: an analysis of 57 patients in Japan. Br J Haematol. 2006;135:362–6.
Wallace WHB, Kelsey TW. Follicle stimulating hormone is an accurate predictor of azoospermia in childhood cancer survivors. PLoS One. 2010;12:e0181377.
Green DM, Sklar CA, Boice JD Jr, Mulvihill JJ, Whitton JA, Stovall M, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the childhood cancer survivor study. J Clin Oncol. 2009;27:2374–81.
Ishida S, Doki N, Shingai N, Yoshioka K, Kakihana K, Sakamaki H, et al. The clinical features of fatal cyclophosphamide-induced cardiotoxicity in a conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Ann Hematol. 2016;95:1145–50.
Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant. 2001;28:283–7.
Teepen JC, van Leeuwen FE, Tissing WJ, van Dulmen-den Broeder E, van den Heuvel-Eibrink MM, van der Pal HJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: role of chemotherapy. J Clin Oncol. 2017;35:2288–98.
Atsuta Y, Hirakawa A, Nakasone H, Kurosawa S, Oshima K, Sakai R, et al. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:1702–9.
Sakai R, Taguri M, Oshima K, Mori T, Ago H, Adachi S, et al. A comparison of tacrolimus and cyclosporine combined with methotrexate for graft-versus-host disease prophylaxis, stratified by stem cell source: a retrospective nationwide survey. Int J Hematol. 2016;103:322–33.
Nasu R, Nannya Y, Shinohara A, Ichikawa M, Kurokawa M. Favorable outcomes of tacrolimus compared with cyclosporine A for GVHD prophylaxis in HSCT for standard-risk hematological diseases. Ann Hematol. 2014;93:1215–23.
Inamoto Y, Flowers ME, Wang T, Urbano-Ispizua A, Hemmer MT, Cutler CS, et al. Tacrolimus versus cyclosporine after hematopoietic cell transplantation for acquired aplastic anemia. Biol Blood Marrow Transplant. 2015;21:1776–82.
Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54.
Iguchi A, Terashita Y, Sugiyama M, Ohshima J, Sato TZ, Cho Y, et al. Graft-versus-host disease (GVHD) prophylaxis by using methotrexate decreases pre-engraftment syndrome and severe acute GVHD, and accelerates engraftment after cord blood transplantation. Pediatr Transplant. 2016;20:114–9.
Acknowledgements
We thank Mrs. Yumiko Shinohe and Mrs. Miyuki Yanagida for their excellent assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iguchi, A., Cho, Y., Yabe, H. et al. Long-term outcome and chimerism in patients with Wiskott–Aldrich syndrome treated by hematopoietic cell transplantation: a retrospective nationwide survey. Int J Hematol 110, 364–369 (2019). https://doi.org/10.1007/s12185-019-02686-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02686-y