Abstract
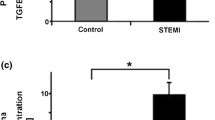
Soluble forms of platelet membrane proteins are released upon platelet activation. We previously reported that soluble C-type lectin-like receptor 2 (sCLEC-2) is released as a shed fragment (Shed CLEC-2) or as a whole molecule associated with platelet microparticles (MP-CLEC-2). In contrast, soluble glycoprotein VI (sGPVI) is released as a shed fragment (Shed GPVI), but not as a microparticle-associated form (MP-GPVI). However, mechanism of sCLEC-2 generation or plasma sCLEC-2 has not been fully elucidated. Experiments using metalloproteinase inhibitors/stimulators revealed that ADAM10/17 induce GPVI shedding, but not CLEC-2 shedding, and that shed CLEC-2 was partially generated by MMP-2. Although MP-GPVI was not generated, it was generated in the presence of the ADAM10 inhibitor. Moreover, antibodies against the cytoplasmic or extracellular domain of GPVI revealed the presence of the GPVI cytoplasmic domain, but not the extracellular domain, in the microparticles. These findings suggest that most of the GPVI on microparticles are induced to shed by ADAM10; MP-GPVI is thus undetected. Plasma sCLEC-2 level was 1/32 of plasma sGPVI level in normal subjects, but both soluble proteins significantly increased in plasma of patients with acute coronary syndrome. Thus, sCLEC-2 and sGPVI are released by different mechanisms and released in vivo upon platelet activation.





Similar content being viewed by others
References
Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–61.
Gurney D, Lip GY, Blann AD. A reliable plasma marker of platelet activation: does it exist? Am J Hematol. 2002;70:139–44.
Nomura S. Extracellular vesicles and blood diseases. Int J Hematol. 2017;105:392–405.
Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–9.
Suzuki-Inoue K, Osada M, Ozaki Y. Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: partners from in utero to adulthood. J Thromb Haemost. 2017;15:219–29.
Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001.
Christou CM, Pearce AC, Watson AA, Mistry AR, Pollitt AY, Fenton-May AE, et al. Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem J. 2008;411:133–40.
Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:189–98.
Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–70.
Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285:24494–507.
Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–60.
Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28:749–55.
Gitz E, Pollitt AY, Gitz-Francois JJ, Alshehri O, Mori J, Montague S, et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood. 2014;124:2262–70.
Kazama F, Nakamura J, Osada M, Inoue O, Oosawa M, Tamura S, et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets. 2015;26:711–9.
Aota T, Naitoh K, Wada H, Yamashita Y, Miyamoto N, Hasegawa M, et al. Elevated soluble platelet glycoprotein VI is a useful marker for DVT in postoperative patients treated with edoxaban. Int J Hematol. 2014;100:450–6.
Inoue O, Suzuki-Inoue K, Shinoda D, Umeda Y, Uchino M, Takasaki S, et al. Novel synthetic collagen fibers, poly(PHG), stimulate platelet aggregation through glycoprotein VI. FEBS Lett. 2009;583:81–7.
Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–52.
Dohi T, Miyauchi K, Ohkawa R, Nakamura K, Kishimoto T, Miyazaki T, et al. Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin Chim Acta. 2012;413:207–12.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Gardiner EE, Karunakaran D, Shen Y, Arthur JF, Andrews RK, Berndt MC. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J Thromb Haemost. 2007;5:1530–7.
Al-Tamimi M, Tan CW, Qiao J, Pennings GJ, Javadzadegan A, Yong AS, et al. Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood. 2012;119:4311–20.
Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J Exp Med. 1998;188:267–76.
Wijeyewickrema LC, Gardiner EE, Moroi M, Berndt MC, Andrews RK. Snake venom metalloproteinases, crotarhagin and alborhagin, induce ectodomain shedding of the platelet collagen receptor, glycoprotein VI. Thromb Haemost. 2007;98:1285–90.
Bender M, Hofmann S, Stegner D, Chalaris A, Bosl M, Braun A, et al. Differentially regulated GPVI ectodomain shedding by multiple platelet-expressed proteinases. Blood. 2010;116:3347–55.
Reinboldt S, Wenzel F, Rauch BH, Hohlfeld T, Grandoch M, Fischer JW, et al. Preliminary evidence for a matrix metalloproteinase-2 (MMP-2)-dependent shedding of soluble CD40 ligand (sCD40L) from activated platelets. Platelets. 2009;20:441–4.
Naitoh K, Hosaka Y, Honda M, Ogawa K, Shirakawa K, Furusako S. Properties of soluble glycoprotein VI, a potential platelet activation biomarker. Platelets. 2015;26:745–50.
Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129:12–23.
Seizer P, May AE. Platelets and matrix metalloproteinases. Thromb Haemost. 2013;110:903–9.
Al-Tamimi M, Mu FT, Moroi M, Gardiner EE, Berndt MC, Andrews RK. Measuring soluble platelet glycoprotein VI in human plasma by ELISA. Platelets. 2009;20:143–9.
Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–9.
Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360.
Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, et al. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–12.
Bigalke B, Potz O, Kremmer E, Geisler T, Seizer P, Puntmann VO, et al. Sandwich immunoassay for soluble glycoprotein VI in patients with symptomatic coronary artery disease. Clin Chem. 2011;57:898–904.
Onselaer MB, Hardy AT, Wilson C, Sanchez X, Babar AK, Miller JLC, et al. Fibrin and D-dimer bind to monomeric GPVI. Blood Adv. 2017;1:1495–504.
Facey A, Pinar I, Arthur JF, Qiao J, Jing J, Mado B, et al. A-disintegrin and metalloproteinase (adam) 10 activity on resting and activated platelets. Biochemistry. 2016;55:1187–94.
Al-Tamimi M, Arthur JF, Gardiner E, Andrews RK. Focusing on plasma glycoprotein VI. Thromb Haemost. 2012;107:648–55.
Zhang X, Zhang W, Wu X, Li H, Zhang C, Huang Z, et al. Prognostic significance of plasma CLEC-2 (C-type lectin-like receptor 2) in patients with acute ischemic stroke. Stroke. 2018. https://doi.org/10.1161/STROKEAHA.118.022563.
Acknowledgements
We thank Hideyuki Tanaka, Chiaki Komatsu, and Hisaichiro Nakazawa for their help of the study. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (M.O.) and the Japan Society for the Promotion of Science (JSPS) through the Funding Program for Next Generation World-leading Researchers (NEXT Program, LS-052) (K.S-I.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Junya Nakamura and Mitsuru Oosawa are an ex-employee and an employee of LSI Medicine Corporation, respectively. Yukio Ozaki, Katsue Suzuki-Inoue, Junya Nakamura, and Mitsuru Oosawa have a patent related to this report (patent no. 6078845) in Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



About this article
Cite this article
Inoue, O., Osada, M., Nakamura, J. et al. Soluble CLEC-2 is generated independently of ADAM10 and is increased in plasma in acute coronary syndrome: comparison with soluble GPVI. Int J Hematol 110, 285–294 (2019). https://doi.org/10.1007/s12185-019-02680-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02680-4