Abstract
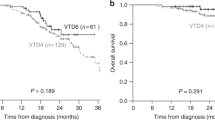
In novel agent era, the impact of immunoparesis at diagnosis on outcomes in symptomatic multiple myeloma (MM) remains unclear. We reviewed medical records of 147 MM patients at Beijing Chao Yang hospital. Most patients exhibited immunoparesis at diagnosis (84%). After a median follow-up of 27 months (range 1–78 months), in the group with immunoparesis at diagnosis, there was a very significantly shorter progression-free survival (PFS) than in the group without immunoparesis (estimated PFS of not reached vs 25 months, P = 0.001). Patients with suppressed Immunoglobulins (Igs) had the tendency to have a shorter OS than patients without suppression (estimated OS of not reached vs 38 months, P = 0.06). In multivariate analysis, the negative impact of immunoparesis on PFS was confirmed. In addition, achievement of both at least VGPR and at least CR was significantly higher in patients with preserved uninvolved Igs than in those with suppression of at least one uninvolved Ig. However, the negative impact of immunoparesis on response was not confirmed in a multivariate analysis. These results suggest immunoparesis in patients with symptomatic MM at diagnosis is an independent poor prognostic factor for upfront bortezomib-containing regimen.

Similar content being viewed by others
References
Kastritis E, Zagouri F, Symeonidis A, Roussou M, Sioni A, Pouli A, et al. Preserved levels of uninvolved immunoglobulins are independently associated with favorable outcome in patients with symptomatic multiple myeloma. Leukemia. 2014;28:2075–9.
Kawano M, Iwato K, Asaoku H, Tanabe O, Tanaka H, Ishikawa H, et al. Altered cytokine activities are related to the suppression of synthesis of normal immunoglobulin in multiple myeloma. Am J Hematol. 1989;30:91–6.
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
Peltonen S, Wasastjerna C, Wager O. Clinical features of patients with a serum M component. Acta Med Scand. 1978;203:257–63.
Alexanian R, Migliore PJ. Normal immunoglobulins in multiple myeloma: effect of melphalan chemotherapy. J Lab Clin Med. 1970;75:225–33.
Pruzanski W, Gidon MS, Roy A. Suppression of polyclonal immunoglobulins in multiple myeloma: relationship to the staging and other manifestations at diagnosis. Clin Immunol Immunopathol. 1980;17:280–6.
Katodritou E, Terpos E, Symeonidis AS, Pouli A, Kelaidi C, Kyrtsonis MC, et al. Clinical features, outcome, and prognostic factors for survival and evolution to multiple myeloma of solitary plasmacytomas: a report of the Greek myeloma study group in 97 patients. Am J Hematol. 2014;89:803–8.
Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–92.
Ludwig H, Milosavljevic D, Berlanga O, Zojer N, Hubl W, Fritz V, et al. Suppression of the noninvolved pair of the myeloma isotype correlates with poor survival in newly diagnosed and relapsed/refractory patients with myeloma. Am J Hematol. 2016;91:295–301.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Ross FM, Avet-Loiseau H, Ameye G, Gutierrez NC, Liebisch P, O’Connor S, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97:1272–7.
Sari M, Sari S, Nalcaci M. The effect of suppressed levels of uninvolved immunoglobulins on the prognosis of symptomatic multiple myeloma. Turk J Haematol. 2017;34:131–6.
Sorrig R, Klausen TW, Salomo M, Vangsted AJ, Frolund UC, Andersen KT, et al. Immunoparesis in newly diagnosed Multiple Myeloma patients: Effects on overall survival and progression free survival in the Danish population. PLoS One. 2017;12:e0188988.
Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50.
Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975–87.
Tsujimoto T, Lisukov IA, Huang N, Mahmoud MS, Kawano MM. Plasma cells induce apoptosis of pre-B cells by interacting with bone marrow stromal cells. Blood. 1996;87:3375–83.
Acknowledgements
The authors would like to thank for these MM patients in the study.
Funding
This project was supported by grant and contract from Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding support (code: XMLX201847).
Author information
Authors and Affiliations
Contributions
WG and JL collected and analyzed data and wrote the manuscript. YJ, GZY, YW, YCL, YL, AJL, YT, HJW, GRW, HXZ, and ZYZ contributed with treatment of patients and reviewed and approved the manuscript. WMC contributed with study design, data collection and interpretation, and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
About this article
Cite this article
Gao, W., Li, J., Jian, Y. et al. Immunoparesis in symptomatic multiple myeloma at diagnosis affects PFS with bortezomib-containing induction therapy, but not ASCT consolidation. Int J Hematol 109, 169–174 (2019). https://doi.org/10.1007/s12185-018-2547-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2547-7