Abstract
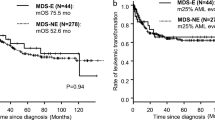
Myelodysplastic syndrome (MDS) is a group of clonal stem cell disorders characterized by hematopoietic insufficiency. The accurate risk stratification of patients with MDS is essential for selection of appropriate therapies. We herein conducted a retrospective cohort study to examine the prognostic value of periodic acid-Schiff (PAS) reaction-positive erythroblasts in MDS patients. We examined the PAS positivity of the bone marrow erythroblasts of 144 patients newly diagnosed with MDS; 26 (18.1%) of them had PAS-positive erythroblasts, whereas 118 (81.9%) did not. The PAS-positive group showed significantly poorer karyotypes as defined in the revised International Prognostic Scoring System (IPSS-R) and higher scores in age-adjusted IPSS-R (IPSS-RA) than the PAS-negative group. Overall survival (OS) and leukemia-free survival (LFS) were also significantly shorter in the PAS-positive group than in the PAS-negative group. Similar results were obtained when only high- and very high risk groups were analyzed using IPSS-RA. This retrospective study suggested that the PAS positivity of erythroblasts is an additional prognostic factor combined with other risk scores for OS and LFS in MDS, and our results may contribute to improved clinical decision-making and rapid risk stratification.




Similar content being viewed by others
References
Garcia-Manero G. Myelodysplastic syndromes: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90:831–41.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008.
Jonas BA, Greenberg PL. MDS prognostic scoring systems—past, present, and future. Best Pract. Res. Clin. Haematol. 2015;28:3–13.
Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–85.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Kawabata H, Tohyama K, Matsuda A, Araseki K, Hata T, Suzuki T, et al. Validation of the revised International Prognostic Scoring System in patients with myelodysplastic syndrome in Japan: results from a prospective multicenter registry. Int J Hematol. 2017;106:375–84.
Komrokji RS, Padron E, Lancet JE, List AF. Prognostic factors and risk models in myelodysplastic syndromes. Clin. Lymphoma. Myeloma Leuk. 2013;13(Suppl 2):S295–9.
Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:97–108.
Patnaik MM, Tefferi A. Refractory anemia with ring sideroblasts and RARS with thrombocytosis. Am J Hematol. 2015;90:549–59.
Kuriyama K, Tomonaga M, Matsuo T, Ginnai I, Ichimaru M. Diagnostic significance of detecting pseudo-Pelger-Huët anomalies and micro-megakaryocytes in myelodysplastic syndrome. Br J Haematol. 1986;63:665–9.
Xiong B, Tang ZH, Zou P, Yue QF, Chen WX, Liu XY. Dysplasia features of myelodysplastic syndrome in ethnically Chinese people. Acta Haematol. 2014;131:126–32.
Verburgh E, Achten R, Louw VJ, Brusselmans C, Delforge M, Boogaerts M, et al. A new disease categorization of low-grade myelodysplastic syndromes based on the expression of cytopenia and dysplasia in one versus more than one lineage improves on the WHO classification. Leukemia. 2007;21:668–77.
Della Porta MG, Travaglino E, Boveri E, Ponzoni M, Malcovati L, Papaemmanuil E, et al. Minimal morphological criteria for defining bone marrow dysplasia: a basis for clinical implementation of WHO classification of myelodysplastic syndromes. Leukemia. 2015;29:66–75.
Giagounidis A, Haase D. Morphology, cytogenetics and classification of MDS. Best Pract. Res. Clin. Haematol. 2013;26:337–53.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Chun K, Hagemeijer A, Iqbal A, Slovak ML. Implementation of standardized international karyotype scoring practices is needed to provide uniform and systematic evaluation for patients with myelodysplastic syndrome using IPSS criteria: an International Working Group on MDS Cytogenetics Study. Leuk Res. 2010;34:160–5.
McManus JFA. Histological demonstration of mucin after periodic acid. Nature. 1946;158:202–22.
Oguro M, Kasahara O, Oshima H. [Simple method for the PAS reaction in blood smear specimens, with reference to lymphocytes]. Rinsho Byori. 1968;16:846–8.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Miesner M, Haferlach C, Bacher U, Weiss T, Macijewski K, Kohlmann A, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood. 2010;116:2742–51.
Rozman M, Navarro J-T, Arenillas L, Aventín A, Giménez T, Alonso E, et al. Multilineage dysplasia is associated with a poorer prognosis in patients with de novo acute myeloid leukemia with intermediate-risk cytogenetics and wild-type NPM1. Ann Hematol. 2014;93:1695–703.
Haferlach T. Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML. J Clin Oncol. 2003;21:256–65.
Goasguen JE, Matsuo T, Cox C, Bennett JM. Evaluation of the dysmyelopoiesis in 336 patients with de novo acute myeloid leukemia: major importance of dysgranulopoiesis for remission and survival. Leukemia. 1992;6:520–5.
Wislocki GB, Rheingold JJ, Dempsey EW. The occurrence of the periodic acid-Schiff reaction in various normal cells of blood and connective tissue. Blood. 1949;4:562–8.
Greig HB, Metz J. The periodic-acid-Schiff reaction as a diagnostic aid in thalassaemia. S Afr J Med Sci. 1957;22:7–12.
Kass L. Periodic acid-schiff-positive megaloblasts in pernicious anemia. Am J Clin Pathol. 1977;67:371–3.
Søndergaard-Petersen H. The Di Guglielmo syndrome: a study of 17 cases. II. Periodic-acid schiff staining of the erythroblasts. Acta Med Scand. 1975;198:175–82.
Carpani G, Rosti A, Cori P, Buscaglia M, Molteni F, Cappati C, et al. Periodic acid Schiff (PAS) positivity in fetal erythroblasts. Haematologica. 1991;76:162–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Masuda, K., Shiga, S., Kawabata, H. et al. PAS positivity of erythroid precursor cells is associated with a poor prognosis in newly diagnosed myelodysplastic syndrome patients. Int J Hematol 108, 30–38 (2018). https://doi.org/10.1007/s12185-018-2443-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2443-1