Abstract
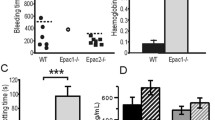
Von Willebrand factor (VWF) is synthesized in megakaryocytes and endothelial cells (ECs) and has two main roles: to carry and protect coagulation factor VIII (FVIII) from degradation by forming VWF–FVIII complex; and to mediate platelet adhesion and aggregation at sites of vascular injury. Previous research using the HEK293 cell line revealed that the VWF K1362 mutation interacted directly with platelet glycoprotein Ib (GPIb). Vwf K1362A knock-in (KI) mice were therefore generated to verify the in vivo function of residue 1362 in binding to platelet GPIb. The Cre-loxP system was employed to introduce the Vwf K1362A mutation systemically in mice. In blood coagulation analysis, the VWF antigen (VWF:Ag) of Lys1362Ala KI homozygous (homo) mice was below the sensitivity of detection by enzyme-linked immunosorbent assay. FVIII activities (FVIII:C) were 47.9 ± 0.3 and 3.3 ± 0.3% (K1362A heterozygous (hetero) and K1362A KI homo mice, respectively) compared to wild-type mice. Immunohistochemical staining analysis revealed that VWF protein did not exist in ECs of K1362A KI homo mice. These results indicated that VWF protein synthesis of K1362A was impaired after transcription in mice. K1362 seems to represent a very important position not only for VWF function, but also for VWF synthesis in mice.






Similar content being viewed by others
References
Handa M, Titani K, Holland LZ, Roberts JR, Ruggeri ZM. The von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Characterization by monoclonal antibodies and partial amino acid sequence analysis of proteolytic fragments. J Biol Chem. 1986;261:12579–85.
Vicente V, Houghten RA, Ruggeri ZM. Identification of a site in the alpha chain of platelet glycoprotein Ib that participates in von Willebrand factor binding. J Biol Chem. 1990;265:274–80.
Murata M, Ware J, Ruggeri ZM. Site-directed mutagenesis of a soluble recombinant fragment of platelet glycoprotein Ib alpha demonstrating negatively charged residues involved in von Willebrand factor binding. J Biol Chem. 1991;266:15474–80.
Scott JP, Montgomery RR, Retzinger GS. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von Willebrand factor-dependent agglutination of platelets. J Biol Chem. 1991;266:8149–55.
Andrews RK, Booth WJ, Gorman JJ, Castaldi PA, Berndt MC. Purification of botrocetin from Bothrops jararaca venom. Analysis of the botrocetin-mediated interaction between von Willebrand factor and the human platelet membrane glycoprotein Ib-IX complex. Biochemistry. 1989;28:8317–26.
Fukuda K, Doggett T, Laurenzi IJ, Liddington RC, Diacovo TG. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat Struct Mol Biol. 2005;12:152–9.
Matsushita T, Sadler JE. Identification of amino acid residues essential for von Willebrand factor binding to platelet glycoprotein Ib. Charged-to-alanine scanning mutagenesis of the A1 domain of human von Willebrand factor. J Biol Chem. 1995;270:13406–14.
Matsushita T, Meyer D, Sadler JE. Localization of von willebrand factor-binding sites for platelet glycoprotein Ib and botrocetin by charged-to-alanine scanning mutagenesis. J Biol Chem. 2000;275:11044–9.
Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–8.
Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, et al. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science (New York, NY). 2002;297:1176–9.
Pendu R, Christophe OD, Denis CV. Mouse models of von Willebrand disease. J Thromb Haemost. 2009;7(Suppl 1):61–4.
Chen J, Zhou H, Diacovo A, Zheng XL, Emsley J, Diacovo TG. Exploiting the kinetic interplay between GPIbalpha-VWF binding interfaces to regulate hemostasis and thrombosis. Blood. 2014;124:3799–807.
Emsley J, Cruz M, Handin R, Liddington R. Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J Biol Chem. 1998;273:10396–401.
Hommais A, Stépanian A, Fressinaud E, Mazurier C, Meyer D, Girma JP, et al. Mutations C1157F and C1234 W of von Willebrand factor cause intracellular retention with defective multimerization and secretion. J Thromb Hemost. 2006;4:148–57.
Acknowledgements
We would like to thank NPO Biotechnology Research and Development for technical assistance. We wish to thank the staff of the Division of Experimental Animals at Nagoya University Graduate School of Medicine for their technical support. We are also grateful to Kimiko Sannodo for obtaining blood from mice and PCR typing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research was supported by JSPS KAKENHI Grant Number JP 22591059 and Novartis Research Grants.
About this article
Cite this article
Sanda, N., Suzuki, N., Suzuki, A. et al. Vwf K1362A resulted in failure of protein synthesis in mice. Int J Hematol 107, 428–435 (2018). https://doi.org/10.1007/s12185-017-2394-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2394-y