Abstract
In the ongoing, international, phase 3 study Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd), nilotinib 300 and nilotinib 400 mg, both twice daily, are compared with imatinib 400 mg once daily for the treatment of newly diagnosed chronic myeloid leukemia in the chronic phase (CML-CP). Results for the overall population in ENESTnd (n = 846) showed that nilotinib resulted in higher response rates vs. imatinib and was well tolerated. Outcomes among Japanese patients in ENESTnd were specifically analyzed after 1 year of follow-up, and showed similar trends to the overall population; we present updated analysis of the Japanese subgroup based on 5 years of follow-up. Among Japanese patients in the nilotinib 300-mg (n = 29), nilotinib 400-mg (n = 23), and imatinib (n = 25) arms, 86.2, 78.3, and 60.0%, respectively, achieved major molecular response [BCR-ABL1 ≤ 0.1% on the International Scale (BCR-ABL1 IS)] by 5 years, and 65.5, 69.6, and 40.0%, respectively, achieved MR4.5 (BCR-ABL1 IS ≤ 0.0032%). Safety results were consistent with prior reports. In this subgroup, one death occurred during treatment in the nilotinib 400-mg twice-daily arm (unknown cause), and one patient in each arm progressed to accelerated phase/blast crisis by the data cutoff.
Similar content being viewed by others
Introduction
Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) is an ongoing pivotal study comparing the efficacy and safety of the second-generation BCR-ABL1 tyrosine kinase inhibitor (TKI) nilotinib vs. the first-generation TKI imatinib as frontline therapy in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) [1]. Upon enrollment in ENESTnd, patients were randomized to receive nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, or imatinib 400 mg once daily. The primary endpoint of ENESTnd was the rate of major molecular response [MMR, defined as BCR-ABL1 ≤ 0.1% on the International Scale (BCR-ABL1 IS)] at 12 months. The trial met its primary endpoint, with a significantly higher rate of MMR in both nilotinib arms (nilotinib 300 mg twice daily, 44%; nilotinib 400 mg twice daily, 43%) than in the imatinib arm (22%; P < .001) [1]. Based on the results from ENESTnd, nilotinib 300 mg twice daily was approved for use as a frontline therapy in patients with newly diagnosed CML-CP [1, 2].
Japan is one of the several countries participating in ENESTnd [1]. For careful evaluation of the safety and efficacy of nilotinib in Japanese patients with newly diagnosed CML-CP, a subgroup analysis of Japanese patients enrolled in ENESTnd was conducted at the time of the primary analysis at 12 months. Similar to the results for the overall ENESTnd population, results for the Japanese subgroup analysis showed higher response rates with nilotinib vs. imatinib and good tolerability [3]. Rates of MMR at 12 months in the Japanese subgroup in ENESTnd were 57, 50, and 24% in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, and both TKIs showed good tolerability [3].
Long-term data from ENESTnd are now available and have consistently shown a positive benefit/risk balance for nilotinib [4, 5]. With long-term follow-up in the overall ENESTnd population, nilotinib continued to show increased rates of molecular response over imatinib, including increased rates of MR4.5 (BCR-ABL1 IS ≤ 0.0032%) [4, 5]. Nilotinib also showed a reduced risk of CML progression to accelerated phase/blast crisis (AP/BC) and CML-related death vs. imatinib [4, 5]. With long-term follow-up, nilotinib has continued to demonstrate good tolerability; however, cardiovascular events (CVEs) have been reported more frequently in the nilotinib arms of ENESTnd than in the imatinib arm [4, 5]. Here we present an updated analysis of long-term outcomes in the Japanese subgroup of patients in ENESTnd based on a minimum follow-up of 5 years.
Materials and methods
Patients and treatment
ENESTnd is an ongoing, randomized, phase 3 trial. The eligibility criteria and study design have been described previously [1, 3, 4, 6, 7]. Briefly, patients with newly diagnosed CML-CP and an Eastern Cooperative Oncology Group performance status of ≤ 2 were randomized 1:1:1 to receive nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, or imatinib 400 mg once daily. Randomization was stratified by Sokal risk score. The study is registered at ClinicalTrials.gov (NCT00471497). ENESTnd remains ongoing, with a total planned follow-up duration of 10 years.
Endpoints
Long-term endpoints analyzed included molecular response rates, survival, progression to AP/BC, and safety. Molecular responses were monitored throughout the study at a central laboratory (MolecularMD, Portland, OR, USA) using real-time quantitative polymerase chain reaction standardized to the IS, as described previously [4]. Progression to AP/BC was defined as the earliest event of progression to AP/BC or CML-related death for each patient, including events reported during randomized study treatment and those reported during follow-up after discontinuation of randomized study treatment. Adverse events (AEs) were monitored throughout the study.
Statistical analysis
Molecular response [MMR, MR4 (BCR-ABL1 IS ≤ 0.01%), and MR4.5] rates at selected time points and cumulative rates achieved by selected time points were calculated. Rates of freedom from progression to AP/BC were estimated using the Kaplan–Meier method, with 95% CI using the SE calculated with the Greenwood formula. Patients without reported events of progression to AP/BC or CML-related death were censored at the date of last assessment (if still on study treatment) or date of last contact (if in follow-up after discontinuation of study treatment). Efficacy analyses included all patients randomized to each arm within the Japanese subgroup. Safety analyses included patients who received ≥ 1 dose of study treatment within the Japanese subgroup. All analyses were based on a data cutoff date of September 30, 2013 (minimum follow-up of 5 years).
Ethics and study management
The study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each patient. Approval of the study protocol was obtained from the appropriate review board or ethics committee for each center.
Results
Patients and treatment
Among 282, 281, and 283 patients with newly diagnosed CML-CP randomized to receive nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, or imatinib, respectively, in the ENESTnd trial, 29, 23, and 25, respectively, were Japanese and were included in this subgroup analysis for efficacy (Table 1). Among patients in the Japanese subgroup in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, the median age was 52, 45, and 54 years; Sokal risk scores were low in 48.3, 60.9, and 52.0% of patients, intermediate in 41.4, 30.4, and 40.0%, and high in 10.3, 8.7, and 8.0%. Of note, 1 patient in each arm did not receive ≥ 1 dose of study treatment and was therefore excluded from the safety analyses (however, these 3 patients were included in the efficacy analyses).
The majority of patients in the Japanese subgroup (nilotinib 300 mg twice daily, 79.3%; nilotinib 400 mg twice daily, 69.6%; imatinib, 60.0%) remained on their assigned study treatment at the data cutoff date. The most common reason for discontinuation among Japanese patients in all arms was AEs (nilotinib 300 mg twice daily, 10.3%; nilotinib 400 mg twice daily, 13.0%; imatinib, 16.0%).
Long-term outcomes
One patient in the Japanese subgroup died (due to an unknown cause) during study treatment (in the nilotinib 400-mg twice-daily arm). One Japanese patient in each arm progressed to AP/BC by the data cutoff (including progression events during or after discontinuation of study treatment). The estimated rate (95% CI) of freedom from progression to AP/BC at 5 years was 96.4% (89.6–100) in the nilotinib 300-mg twice-daily arm, 95.0% (85.4–100) in the nilotinib 400-mg twice-daily arm, and 95.8% (87.8–100) in the imatinib arm.
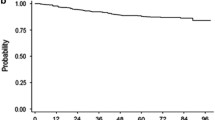
By 5 years, 25 of 29 (86.2%), 18 of 23 (78.3%), and 15 of 25 (60.0%) Japanese patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, achieved MMR or better; 19 (65.5%), 17 (73.9%), and 11 (44.0%) achieved MR4 or better; and 19 (65.5%), 16 (69.6%), and 10 (40.0%) achieved MR4.5 (Fig. 1). At 5 years, 23 of 29 (79.3%), 15 of 23 (65.2%), and 14 of 25 (56.0%) Japanese patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, had MMR or better; 17 (58.6%), 13 (56.5%), and 9 (36.0%) had MR4 or better; and 14 (48.3%), 12 (52.2%), and 9 (36.0%) had MR4.5 (Fig. 2).
Cumulative molecular response rates. The cumulative proportions of patients in the Japanese subgroup with a response of a MMR or better, b MR4 or better, or c MR4.5 detected by each time point are shown. Curves terminate at the latest time point at which all patients who achieved the indicated response level had reached that level
Safety
In the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, 28, 22, and 24 patients in the Japanese subgroup received ≥ 1 dose of study treatment and were included in the safety analyses. The specific AEs leading to discontinuation in the nilotinib 300-mg twice-daily arm were pancytopenia, thrombocytopenia, and blood bilirubin increase (n = 1 each). The specific AEs leading to discontinuation in the nilotinib 400-mg twice-daily arm were angina pectoris, gastric cancer, and interstitial lung disease (n = 1 each). The specific AEs leading to discontinuation in the imatinib arm were nausea, pancreatitis acute, hyperbilirubinemia, and B-cell lymphoma (n = 1 each).
Safety results in the Japanese subgroup remained consistent with those in the overall ENESTnd safety population [4] and with the prior analysis of the Japanese subgroup [3]. AEs occurring in ≥ 40% of Japanese patients in any arm were nasopharyngitis, rash, headache, back pain, alanine aminotransferase increase, diarrhea, influenza, vomiting, nausea, edema peripheral, and face edema (Table 2). Serious AEs were reported in 7 (25.0%), 8 (36.4%), and 5 (20.8%) Japanese patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively. Serious AEs in the nilotinib 300-mg twice-daily arm included thrombocytopenia in 2 patients (7.1%) and adenocarcinoma, calculus ureteric, cerebral infarction, cholelithiasis, ileus paralytic, intervertebral disc protrusion, pain in extremity, pancytopenia, peripheral artery disease, and prostate cancer in 1 patient (3.6%) each. Serious AEs in the nilotinib 400-mg twice-daily arm included abnormal hepatic function in 2 patients (9.1%) and angina pectoris, angina unstable, death, drug-induced liver injury, gastric cancer, interstitial lung disease, pneumonia, renal impairment, and vomiting in 1 patient (4.5%) each. Serious AEs in the imatinib arm included cellulitis in 2 patients (8.3%) and pancreatitis acute, B-cell lymphoma, contusion, hemothorax, ligament sprain, edema peripheral, pleural effusion, rib fracture, and spontaneous pneumothorax in 1 patient (4.2%) each.
Two Japanese patients (both in the nilotinib 300-mg twice-daily arm; 7.1%) had AEs of hypertension, and 1 Japanese patient (in the nilotinib 400-mg twice-daily arm; 4.5%) had pulmonary hypertension. No Japanese patient in any arm had an AE of thrombophlebitis or superficial thrombophlebitis. Limb venous thrombosis was reported in 1 Japanese patient (in the imatinib arm; 4.2%). To carefully evaluate the rates of key clinically relevant event types, including severe fluid retention, hepatotoxicity, pancreatitis, significant bleeding, and CVEs, the combined frequency of multiple AE terms relevant to each type of event were calculated and are shown in Table 3. Severe fluid retention was more frequent among Japanese patients in the imatinib arm (54.2%) than in either nilotinib arm (nilotinib 300 mg twice daily, 10.7%; nilotinib 400 mg twice daily, 13.6%).
CVEs were reported in 2 Japanese patients in each nilotinib arm and none in the imatinib arm. One ischemic cerebrovascular event and 1 peripheral artery disease event were reported in the Japanese subgroup in the nilotinib 300-mg twice-daily arm. The patient with an ischemic cerebrovascular event (specifically, cerebral infarction) was a 68-year-old man [baseline glycated hemoglobin (HbA1c), 6.4%; total cholesterol, 5.76 mmol/L (222 mg/dL); blood pressure, 131/78 mmHg] receiving treatment for hypertension who had received 4.7 years of nilotinib treatment before the event. The patient with a peripheral artery disease event (right leg chronic artery occlusion) was a 54-year-old man with a history of hyperlipidemia, smoking, and diabetes [baseline HbA1c, 6.6%; total cholesterol, 4.19 mmol/L (162 mg/dL); blood pressure, 126/63 mmHg] who had received 2.0 years of nilotinib treatment before the event. Two Japanese patients in the nilotinib 400-mg twice-daily arm had ischemic heart disease events. Specifically, 1 event was unstable angina, reported in a 60-year-old man with a history of hyperlipidemia and a body mass index of 25.7 kg/m2 [baseline HbA1c, 6.3%; total cholesterol, 4.83 mmol/L (186 mg/dL); blood pressure, 119/78 mmHg] who was receiving treatment for hypertension and who had received 4.3 years of nilotinib treatment before the event. Following the initial diagnosis of unstable angina, the patient was hospitalized and underwent percutaneous coronary intervention on 4 occasions within 7 months; on the fourth occasion, the patient also received a stent. The other ischemic heart disease event was angina pectoris of 3-vessel disease that required coronary artery bypass graft surgery, reported in a 35-year-old man without any risk factors for ischemic heart disease except for a history of smoking [baseline HbA1c, 5.6%; total cholesterol, 4.78 mmol/L (185 mg/dL); blood pressure, 102/71 mmHg], who had received 2.4 years of nilotinib treatment before the event. Following the CVE, the patient switched to imatinib; after 4.2 years of imatinib therapy, a diagnosis of peripheral artery disease was made, but it was not clear whether its onset was during the nilotinib treatment.
The frequencies of treatment-emergent cholesterol and HbA1c elevations above clinically relevant thresholds [8,9,10] were evaluated among patients in the Japanese subgroup. Treatment-emergent elevations in total cholesterol above clinically relevant thresholds were more common in the nilotinib arms than in the imatinib arm (Table 4). In contrast, similar proportions of patients in all 3 arms developed low-density lipoprotein elevations of > 2.58 mmol/L (100 mg/dL), although elevations of > 1.81 or > 4.91 mmol/L (70 or 190 mg/dL) were more common in the nilotinib arms. Treatment-emergent HbA1c elevations above the thresholds diagnostic of prediabetes (> 5.7%) or diabetes (≥ 6.5%) were more common in both nilotinib arms than in the imatinib arm.
Discussion
With 5 years of follow-up, nilotinib continued to result in higher rates of MMR vs. imatinib in Japanese patients with newly diagnosed CML-CP; in addition, nilotinib resulted in higher rates of deep molecular responses than imatinib. These results are consistent with those from the 5-year analysis of the overall ENESTnd population, in which 77.0, 77.2, and 60.4% of patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, achieved MMR by 5 years; 65.6, 63.0, and 41.7% achieved MR4; and 53.5, 52.3, and 31.4% achieved MR4.5 (for the overall population, nominal P < .0001 for each nilotinib arm vs. imatinib at each response level using the Cochran–Mantel–Haenszel test stratified by Sokal risk group) [4]. A post hoc analysis of the overall ENESTnd population also showed that nilotinib resulted in higher rates of sustained deep molecular response vs. imatinib, potentially leading to higher rates of eligibility for attempting treatment-free remission (TFR) with nilotinib over the TFR-eligibility rates achieved with imatinib [11].
Safety results remained consistent with prior reports [1, 3, 4, 6, 7]. The most frequent AEs in the Japanese subgroup were generally similar to those in the overall ENESTnd population [4]. Nasopharyngitis, rash, and headache were among the most frequent AEs in the nilotinib arms both overall and in the Japanese subgroup, although the frequencies of each of these AEs among nilotinib-treated patients tended to be numerically higher in the Japanese subgroup vs. the overall population. The biggest difference was in rates of nasopharyngitis, which was reported approximately 3 times more frequently in the Japanese subgroup (in all 3 arms) vs. the overall population (in the overall ENESTnd population, 26.9, 22.7, and 21.4% of patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, experienced nasopharyngitis by the 5-year analysis vs. 78.6, 72.7, and 66.7%, respectively, in the Japanese subgroup) [4]; however, this difference may be the result of an increased reporting of nasopharyngitis by Japanese clinicians due to the fact that they frequently administer prescription medications for mild cases of the common cold.
Second-generation TKIs, including nilotinib, are associated with higher rates of CVEs than imatinib [4, 12,13,14]. With 5 years of follow-up in ENESTnd, the rates of CVEs in the Japanese subgroup (nilotinib 300 mg twice daily, 7.1%; nilotinib 400 mg twice daily, 9.1%; imatinib, 0%) were similar to those in the overall population (7.5, 13.4, and 2.1%, respectively) [4]. Overall in the Japanese subgroup, 1 patient experienced an ischemic cerebrovascular event while receiving nilotinib (300-mg twice-daily arm), 1 patient experienced peripheral artery disease while receiving nilotinib (300-mg twice-daily arm), and 2 patients experienced ischemic heart disease while receiving nilotinib (both in the 400-mg twice-daily arm); these results are consistent with those in the overall population in the nilotinib arms, in which ischemic heart disease events were more commonly reported than either ischemic cerebrovascular events or peripheral artery disease. An exploratory analysis in the overall ENESTnd population showed that most patients with CVEs during nilotinib treatment had intermediate or high Framingham general CV risk scores [15] at baseline, whereas those with low Framingham risk at baseline were less likely to develop CVEs [4]. This suggests that it may be possible to identify patients who are at increased CV risk before initiating treatment, although ≥ 1 patient in the current cohort who developed ischemic heart disease would have been considered low risk due to his young age (35 years) and limited risk factors. Thus, all patients should receive proper monitoring and management of CV risk at baseline and during therapy [2]. In the future, elucidation of the mechanism for development of CVEs during nilotinib treatment, together with periodic monitoring of patients treated in clinical practice, is needed.
Nilotinib has been associated with treatment-emergent elevations in cholesterol as well as an increased risk of developing prediabetes or diabetes [4]. In the 5-year analysis of the overall ENESTnd population, treatment-emergent total cholesterol elevations of > 5.17 mmol/L (200 mg/dL) were reported in 51.6, 59.2, and 16.4% of patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively; low-density lipoprotein elevations of > 2.58 mmol/L (100 mg/dL) were reported in 38.4, 43.3, and 18.6% of patients, respectively [4]. In the overall ENESTnd population, patients in the nilotinib arms who initiated statin therapy after developing total cholesterol elevations of > 5.17 mmol/L on study were found to subsequently achieve reductions in median cholesterol levels [4]. This highlights the importance of properly monitoring and managing cholesterol levels and other comorbidities in all patients. In the 5-year analysis of the overall ENESTnd population, treatment-emergent HbA1c levels of > 5.7% (i.e., diagnostic of prediabetes [10]) were reported in 21.9, 24.2, and 18.2% of patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively, and levels of ≥ 6.5% (i.e., diagnostic of diabetes [10]) were reported in 11.8, 11.6, and 3.6% of patients, respectively [4].
Most patients in the Japanese subgroup who were randomized to nilotinib remained on their assigned study treatment at the 5-year data cutoff (nilotinib 300 mg twice daily, 79.3%; nilotinib 400 mg twice daily, 69.6%; imatinib, 60.0%). This is consistent with, and even higher than, the proportion of patients in the overall population who continued receiving their assigned treatment at the same time point (59.9, 61.9, and 49.8%, respectively) [4]. Overall in the Japanese subgroup, only 1 death was reported by the 5-year data cutoff (in the nilotinib 400-mg twice-daily arm), and most patients did not experience progression to AP/BC (1 progression to AP/BC was reported in each arm by the 5-year data cutoff). In conclusion, results from this subgroup analysis continue to support the use of nilotinib 300 mg twice daily in Japanese patients with newly diagnosed CML-CP. However, patients’ individual needs and comorbidities should be considered when selecting a frontline treatment (as described in existing guidelines [16]).
References
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9.
Tasigna (nilotinib) (package insert). East Hanover: Novartis Pharmaceuticals Corporation; 2017.
Nakamae H, Shibayama H, Kurokawa M, Fukuda T, Nakaseko C, Kanda Y, et al. Nilotinib as frontline therapy for patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase: results from the Japanese subgroup of ENESTnd. Int J Hematol. 2011;93:624–32.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, Lobo C, et al. Efficacy and safety of nilotinib vs imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase: 6-year follow-up of ENESTnd. Haematologica. 2015;100(s1):81 (abstract P228).
Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–51.
Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–203.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81.
Stone NJ, Robinson J, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45.
American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40:S1–135.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Taningco L, Deng W, et al. Impact of treatment with frontline nilotinib (NIL) vs imatinib (IM) on sustained deep molecular response (MR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP). Blood. 2015;126(23) (abstract 2781).
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34:2333–40.
Gambacorti-Passerini C, Cortes JE, Lipton JH, Dmoszynska A, Wong RS, Rossiev V, et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol. 2014;89:947–53.
Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:612–21.
D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic myeloid leukemia. v2.2017. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. Accessed 19 Jan 2017.
Acknowledgements
Medical writing assistance for the preparation of this article was provided by Karen Kaluza, Ph.D. (ArticulateScience LLC) and funded by Novartis Pharma KK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following relationships: Hirohisa Nakamae reports research funding from Novartis during the conduct of the study; consulting or advisory role, honoraria, and travel support from Novartis outside the submitted work; Tetsuya Fukuda reports honoraria from Novartis outside the submitted work; Chiaki Nakaseko reports research funding and honoraria from BMS and Pfizer outside the submitted work; honoraria from Novartis outside the submitted work; Yoshinobu Kanda reports personal fees from Novartis during the conduct of the study; personal fees from Dainippon-Sumitomo and Pfizer outside the submitted work; research funding and personal fees from Astellas, Kyowa-Hakko Kirin, Taisho-Toyama, and Merck Sharp & Dohme outside the submitted work; research funding from Chugai, Bristol-Myers Squibb, Takeda, Tanabe Mitsubishi, Toyama Kagaku, Nippon Shinyaku, Alexion, Yakult, Shionogi, Otsuka, and Nippon Kayaku outside the submitted work; Ken Ohmine reports personal fees Takara Bio Inc., Ono Pharmaceutical Co., Ltd., and Kyowa Kirin Pharmaceutical Development Inc. outside the submitted work; Takaaki Ono reports research funding from Celgene, Kyowa Hakko Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Toyama Kagaku, and Merck Sharp & Dohme outside the submitted work; Itaru Matsumura reports research funding and personal fees from Bristol-Myers Squibb and Pfizer Japan Inc. during the conduct of the study; personal fees from Novartis Pharma KK during the conduct of the study; Akira Matsuda reports study fees from Kyowa Hakko Kirin Co., Ltd. and GlaxoSmithKline during the conduct of the study; research grant from Chugai Pharmaceutical Co., Ltd. during the conduct of the study; personal fees from Nippon Shinyaku Co., Ltd., Alexion Pharmaceuticals, Inc., Sanofi KK, GlaxoSmithKline KK, Kyowa Hakko Kirin Co., Ltd., outside the submitted work; Makoto Aoki is employed by Novartis Pharma KK; Kazuo Ito is employed by Novartis Pharma KK; Hirohiko Shibayama reports research funding, consulting or advisory role, honoraria and lecture fees from Novartis during the conduct of the study.
About this article
Cite this article
Nakamae, H., Fukuda, T., Nakaseko, C. et al. Nilotinib vs. imatinib in Japanese patients with newly diagnosed chronic myeloid leukemia in chronic phase: long-term follow-up of the Japanese subgroup of the randomized ENESTnd trial. Int J Hematol 107, 327–336 (2018). https://doi.org/10.1007/s12185-017-2353-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2353-7