Abstract
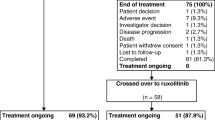
Ruxolitinib, a potent JAK1/JAK2 inhibitor, was found to be superior to the best available therapy (BAT) in controlling hematocrit, reducing splenomegaly, and improving symptoms in the phase 3 RESPONSE study of patients with polycythemia vera with splenomegaly who experienced an inadequate response to or adverse effects from hydroxyurea. We report findings from a subgroup analysis of Japanese patients in RESPONSE (n = 18). The composite response rate (hematocrit control and spleen response) was higher in patients receiving ruxolitinib (50.0%) than in those receiving BAT (8.3%). A total of 50.0% of patients randomized to ruxolitinib achieved a spleen response vs 8.3% of those receiving BAT; 100 and 33.3% of patients in the respective groups achieved hematocrit control, with mean hematocrit in ruxolitinib-treated patients remaining stable at < 45% throughout the study. Similarly, a higher proportion of ruxolitinib-treated patients achieved complete hematologic remission (33.3 vs 16.7%). Ruxolitinib also led to rapid improvements in pruritus. All responses with ruxolitinib were durable to week 80, and its safety profile was consistent with that in the overall study. These findings suggest that ruxolitinib is an effective and well-tolerated treatment option for Japanese patients with polycythemia vera with an inadequate response to or adverse effects from hydroxyurea.






Similar content being viewed by others
References
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51.
Stuart BJ, Viera AJ. Polycythemia vera. Am Fam Physician. 2004;69:2139–44.
Hensley B, Geyer H, Mesa R. Polycythemia vera: current pharmacotherapy and future directions. Expert Opin Pharmacother. 2013;14:609–17.
Passamonti F. How I treat polycythemia vera. Blood. 2012;120:275–84.
Vannucchi AM. Insights into the pathogenesis and management of thrombosis in polycythemia vera and essential thrombocythemia. Intern Emerg Med. 2010;5:177–84.
Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–90.
Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–81.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
Kralovics R, Teo SS, Buser AS, Brutsche M, Tiedt R, Tichelli A, et al. Altered gene expression in myeloproliferative disorders correlates with activation of signaling by the V617F mutation of Jak2. Blood. 2005;106:3374–6.
Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–92.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97.
Levine RL. JAK-mutant myeloproliferative neoplasms. Curr Top Microbiol Immunol. 2012;355:119–33.
Vannucchi AM, Guglielmelli P. JAK2 mutation-related disease and thrombosis. Semin Thromb Hemost. 2013;39:496–506.
Tefferi A. Mutations galore in myeloproliferative neoplasms: would the real Spartacus please stand up? Leukemia. 2011;25:1059–63.
Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Blood. 2014;124:3529–37.
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–8.
Abelsson J, Andréasson B, Samuelsson J, Hultcrantz M, Ejerblad E, Johansson B, et al. Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54:2226–30.
Abdulkarim K, Ridell B, Johansson P, Kutti J, Safai-Kutti S, Andréasson B. The impact of peripheral blood values and bone marrow findings on prognosis for patients with essential thrombocythemia and polycythemia vera. Eur J Haematol. 2011;86:148–55.
Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32.
Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–70.
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33.
Barbui T, Masciulli A, Marfisi MR, Tognoni G, Finazzi G, Rambaldi A, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126:560–1.
Alvarez-Larrán A, Pereira A, Cervantes F, Arellano-Rodrigo E, Hernández-Boluda JC, Ferrer-Marín F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119:1363–9.
Alvarez-Larrán A, Kerguelen A, Hernández-Boluda JC, Pérez-Encinas M, Ferrer-Marín F, Bárez A, et al. Frequency and prognostic value of resistance/intolerance to hydroxycarbamide in 890 patients with polycythaemia vera. Br J Haematol. 2016;172:786–93.
Reiter A, Harrison C. How we identify and manage patients with inadequately controlled polycythemia vera. Curr Hematol Malig Rep. 2016;11:356–67.
Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426–35.
Verstovsek S, Vannucchi AM, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80 week follow up from the RESPONSE trial. Haematologica. 2016;101:821–9.
Dan K, Yamada T, Kimura Y, Usui N, Okamoto S, Sugihara T, et al. Clinical features of polycythemia vera and essential thrombocythemia in Japan: retrospective analysis of a nationwide survey by the Japanese Elderly Leukemia and Lymphoma Study Group. Int J Hematol. 2006;83:443–9.
Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch H, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol. 2010;148:961–3.
Kirito K, Sakamoto M, Enomoto N. Elevation of the hepatitis B virus DNA during the treatment of polycythemia vera with the JAK kinase inhibitor ruxolitinib. Intern Med. 2016;55:1341–4.
Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–52.
Kaifie A, Kirschner M, Wolf D, Maintz C, Hänel M, Gattermann N, et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): analysis from the German SAL-MPN-registry. J Hematol Oncol. 2016;9:18.
Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–72.
Quintás-Cardama A, Abdel-Wahab O, Manshouri T, Kilpivaara O, Cortes J, Roupie AL, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013;122:893–901.
Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126:1762–9.
Alvarez-Larrán A, Pérez-Encinas M, Ferrer-Marin F, Hernández-Boluda JC, Ramírez MJ, Martínez-López J, et al. Risk of thrombosis according to need of phlebotomies in patients with polycythemia vera treated with hydroxyurea. Haematologica. 2017;102:103–9.
Jung CW, Shih LY, Xiao Z, Jie J, Hou HA, Du X, et al. Efficacy and safety of ruxolitinib in Asian patients with myelofibrosis. Leuk Lymphoma. 2015;56:2067–74.
Komatsu N, Kirito K, Shimoda K, Ishikawa T, Ohishi K, Ohyashiki K et al. Assessing the safety and efficacy of ruxolitinib in a multicenter, open-label study in Japanese patients with myelofibrosis. Int J Hematol. 2017;105:309–17.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98.
Al-Ali HK, Griesshammer M, le Coutre P, Waller CF, Liberati AM, Schafhausen P, et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica. 2016;101:1065–73.
Oritani K, Okamoto S, Tauchi T, Saito S, Ohishi K, Handa H, et al. A multinational, open-label, phase 2 study of ruxolitinib in Asian patients with myelofibrosis: Japanese subset analysis. Int J Hematol. 2015;101:295–304.
Acknowledgements
Editorial assistance was provided by Karen Chinchilla, Ph.D., and was funded by Novartis Pharma KK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. K. has received personal fees from Novartis Pharma KK, M. H. has received grants and personal fees from Novartis Pharma KK, K. M. has received personal fees from Novartis Pharmaceuticals Corporation, Nippon Shinyaku Co Ltd, Pfizer Inc, and Alexion Pharmaceuticals, Inc; B. G. is an employee of Novartis Pharmaceuticals Corporation. T. A., K. Y., and K. I. are employees of Novartis Pharma K. K. M. T., H. H., S. O., and K. S. have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kirito, K., Suzuki, K., Miyamura, K. et al. Ruxolitinib is effective and safe in Japanese patients with hydroxyurea-resistant or hydroxyurea-intolerant polycythemia vera with splenomegaly. Int J Hematol 107, 173–184 (2018). https://doi.org/10.1007/s12185-017-2333-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2333-y