Abstract
Purpose of Review
Cardiovascular disease (CVD) is the leading cause of death in women worldwide. Preeclampsia (PE) is an important and acknowledged female-specific risk factor. Women with a history of PE have an elevated risk to develop hypertension in the decade following their index pregnancy. In this paper, we aim to provide recent insights in the future risk of preeclampsia and describe how prevention strategies can be optimized.
Recent Findings
The development of premature hypertension is caused by multiple factors such as genetics and a persistent enhanced inflammatory state after PE. Systemic inflammation accelerates endothelial dysfunction and promotes the process of atherosclerosis. In formerly preeclamptic women, signs of subclinical atherosclerosis are present at young age. Timely cardiovascular screening after PE is recommended; however, the how and when are yet to be determined. Given the large impact of a previous preeclamptic pregnancy on cardiovascular risk, the question arises whether we should not intensify secondary prevention in these high-risk women.
Summary
Obstetric history can provide useful information about CVD risk in women after their reproductive life, especially if they had a pregnancy complicated by preeclampsia. Early detection of hypertension could optimize prevention and treatment strategies and mitigate CVD risk. For high-risk women, preeclampsia may be considered a first cardiovascular event that requires secondary prevention according to guidelines.
Similar content being viewed by others
Introduction
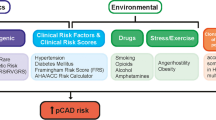
Cardiovascular disease (CVD) is the leading cause of death in women worldwide. In the last two decades, overwhelming evidence has shown that CVD risk is elevated after a pregnancy complicated by preeclampsia (PE) [1,2,3,4]. Several global guidelines, such as the 2011 American College of Cardiology/American Heart Association’s (ACC/AHA) guideline [5], the 2016 European Society of Cardiology (ESC) guideline CVD prevention [6], and the 2017 ACC/AHA guideline on high blood pressure [7], acknowledge preeclampsia as a female-specific cardiovascular risk factor and recommend timely risk monitoring after PE. As such, pregnancy acts as a window for future cardiovascular health [8]. It is yet to establish, however, whether preeclampsia is an independent risk factor for CVD or acts through traditional CVD risk factors [9]. The most common thought is that PE is driven by an enhanced susceptibility for CVD and interacts with environmental factors. This review aims to describe the current knowledge on preeclampsia as a risk factor for CVD later in life.
Preeclampsia and Subsequent Cardiovascular Risk
Preeclampsia is one of the hypertensive disorders of pregnancy (HDP) and accounts for the most severe form. The International Society for the Study of Hypertension (ISSHP) defines preeclampsia as new-onset hypertension after 20 weeks gestation in combination with either proteinuria (≥ 300 mg/day) or other maternal dysfunctions, such as renal insufficiency, liver involvement, neurological or hematological complications, or uteroplacental dysfunction [10]. Preeclampsia complicates 3–5% of all pregnancies, leading to maternal and fetal morbidity caused by growth restriction due to uteroplacental dysfunction. Moreover, it can potentially lead to intrauterine death [11]. In Western countries, such as the Netherlands, maternal mortality is low, but PE remains the most important cause [12].
Hypertension is diagnosed when the systolic blood pressure exceeds 140 mmHg or the diastolic blood pressure exceeds 90 mmHg on two occasions [10, 13]. After a preeclamptic pregnancy, women have at least a twofold increased risk of developing any manifestation of CVD. The risk of developing chronic hypertension is particularly elevated, with a four times higher risk compared with women having had a normotensive pregnancy [1, 2]. Furthermore, several meta-analyses showed a 2.0 to 2.5 elevated risk of developing ischemic heart disease [1, 3, 4]. In addition, the risk of future cerebrovascular disease is twice higher [1, 3], and a fourfold increased risk was observed for developing heart failure (HF) [4].
A retrospective cohort study in over 1.6 million pregnancies demonstrated corresponding risks and showed that cardiovascular complications of pregnancies, such as hypertensive disorders of pregnancy, can identify women at young age being at risk for premature CVD [14•]. In the 2.7 years following the pregnancy complicated by preeclampsia women had a 2.5-fold increased risk for myocardial infarction (MI), a 3.0-fold increased risk for HF, and a 2.3-fold increased risk for stroke, compared with women after normotensive pregnancies [14•]. Women with chronic hypertension with superimposed PE had the highest risk for MI, HF, and stroke.
Cardiovascular Risk Prediction
Common risk factors for both CVD and pregnancy complications such as preeclampsia include obesity, chronic hypertension, diabetes mellitus, renal disease, immune diseases, and black race. Hypertension is the most important risk factor for CVD, cerebrovascular disease, and chronic kidney disease.
Several studies recently showed that the inclusion of HDP in any of the most frequently used CVD risk scores does not improve the prediction of future CVD events [15•, 16, 17]. A limitation of these studies however is that these risk prediction models were tested in women aged 50 years or older, when traditional CVD risk factors emerge as well. Stuart et al. analyzed data of women aged 40 years or older, showing that both HDP and parity were independently associated with CVD at ages 40 to 49 years [15•]. However, the addition of HDP to the atherosclerotic cardiovascular disease (ASCVD) risk score did not result in better risk stratification, as traditional cardiovascular risk factors and risk factors for preeclampsia, or HDP in general, widely overlap. Since systolic blood pressure and use of antihypertensive medication are important predictors in these risk models, these factors may explain the lack of effect of adding HDP to the models. Furthermore, women who have hypertension after PE have a twofold increased risk of developing CVD, compared with women who are normotensive after PE [18].
A discussion is currently evolving on whether we should consider PE and severe HDP not as risk factors for CVD, but as first manifestations of a cardiovascular event for which secondary prevention rules are more appropriate. A recent study showed that women after a pregnancy with PE have a twofold higher risk of identifiable coronary artery calcium (CAC) with CT scans at the age of 50 when compared with a reference population [19••]. This means that signs of subclinical atherosclerosis appear at a young age and many years earlier than usual [20]. As hypertension is the first important risk factor emerging after PE or severe HDP, a stricter blood pressure control than current guidelines indicate in HDP-affected patients may be the first important step forward for this high-risk group. As hypertension determines up to half of an individual’s CVD risk [21], 38% of cardiovascular mortality in women could be prevented by eliminating this risk factor [22]. Moreover, patients with a CVD event in history without hypertension can benefit from antihypertensive treatment to reduce MI, HF, and cerebrovascular disease [23].
Onset of Hypertension After Preeclampsia
Over the last years, several studies have been done to obtain insight in the onset of hypertension and other CVD risk factors after PE [24, 25]. Other CVD risk factors, such as lipid levels, also show a less favorable profile after PE, but these are often determined when women are still premenopausal and flattered by their still high estrogen levels [26].
The timing to start screening for hypertension is still debated. Various studies showed that approximately 40% of women with early-onset PE had hypertension 10 years after pregnancy [24, 27]. This is in line with a more recent study by Heida et al. [28] that showed a mean age of 43.5 years at onset of chronic hypertension after HDP. A few studies [29,30,31] showed the strongest risks for development of chronic hypertension in the first 5 years after pregnancy. In one study, 41.5% of women already had hypertension 1 year after severe PE, of which 17.5% had masked hypertension [32]. These findings highlight the shortcomings of incidental in-office blood pressure measurements and request other ways of measuring blood pressure, such as ambulatory blood pressure monitoring or home blood pressure monitoring.
As hypertension develops 10–15 years earlier in women after PE compared with women after normotensive pregnancies, screening from at least 35 years of age onwards is proposed [26]. Prepregnancy risk factors, such as family history of hypertension, obesity, and elevated blood pressure, are all important in the onset of hypertension and other CVD risk factors after pregnancy [33,34,35]. In a prospective cohort study, however, Egeland et al. [36] showed that the increased risk of hypertension persisted after adjustment for prepregnancy risk factors.
Enhanced Inflammation After Previous Preeclampsia
During PE, several markers of inflammation (cytokines) are substantially increased in the maternal circulation and the placenta, resulting in chronic systemic and local placental inflammation, which contributes to the complications that occur during PE. The most prominent markers are tumor necrosis factor α (TNF-α), interleukin (IL) 6, and IL-17 [37]. TNF-α signaling results in endothelial cell activation and a reduction in nitric oxide synthase (NOS), leading to endothelial dysfunction that explains all maternal clinical features. The levels of vasoconstrictor endothelin 1 (ET-1) are also increased. In contrast, the levels of IL-10, an anti-inflammatory marker that inhibits the activation of interferon γ (IFN-γ), IL-2, and TNF-α, are decreased in preeclamptic women [38]. This imbalance between proinflammatory and regulatory cytokines during a preeclamptic pregnancy suggests that PE is a state of dysregulated immune activation.
Women with a history of PE have alterations in several miscellaneous markers (TNF-α, IL-6, leptin, adiponectin, homocysteine, SE-selectin, and pregnancy-associated plasma protein-A (PAPP-A)) at 8–10 years after the index pregnancy compared with control subjects [39, 40]. Higher baseline levels of C-reactive protein (CRP), IL-6, and fibrinogen have been observed months to years after delivery in previously preeclamptic women [41]. An increase in the CRP response to vaccination and a consistent pattern of increased acute-phase responses (APRs) to vaccination for all inflammatory markers were also found among women after PE compared with controls, indicating that vascular responses have changed after PE [42]. This effect was independent of the traditional cardiovascular risk factors, which further enforces the concept that a preeclamptic pregnancy may be considered a first vascular event. However, not all women affected by PE seem to have an equally elevated CVD risk. It remains to be elucidated whether women with an elevated proinflammatory state after PE are at the highest risk of developing CVD in the years thereafter.
Signs of Subclinical Atherosclerosis After Preeclampsia
Systemic inflammation accelerates endothelial dysfunction, atherosclerosis, and premature arterial stiffness [43]. Therefore, the enhanced inflammatory state after a preeclamptic pregnancy may induce hypertension and subclinical atherosclerosis. A widely used non-invasive measure to evaluate subclinical atherosclerosis is carotid intima-media thickness (cIMT) [44]. An increased cIMT value is a predictor of coronary artery disease in both pre- and postmenopausal women [45]. Two recent meta-analyses showed that cIMT values are higher in women after preeclampsia compared with those in women experiencing a normotensive pregnancy, both at time of pregnancy and in the first 10 years postpartum [46, 47].
In a review on shared pathophysiological mechanisms, De Jager and colleagues [48] showed that many common determinants are involved in both PE and atherosclerotic plaque erosions. Especially, IL-6 and TNF-α are major contributors to the inflammatory response in these conditions.
The shared inflammatory response in PE and atherosclerotic disease and the elevated cIMT values all suggest that formerly preeclamptic women have an increased risk of having subclinical coronary atherosclerosis at a younger age.
In 2018, the first study was published that compared the prevalence of coronary atherosclerosis using coronary artery calcification (CAC) score in women aged 45 to 55 years [19••]. Thirty percent of formerly preeclamptic women showed signs of CAC around the age of 50 years compared with 18% in the reference group [19••]. However, women with a history of PE also had hypertension more often, which may have contributed to the higher CAC scores. When having an acute coronary syndrome (ACS), nearly all women with a previous preeclampsia had chronic hypertension [49].
Preeclampsia Should Be Considered a First Cardiovascular Event
Since CVD risk prediction models are not suitable to distinguish high-risk women after PE adequately, we have to identify better which women after PE are at highest risk. For those with an enhanced inflammatory state and/or signs of subclinical atherosclerosis, we should seriously consider to apply our secondary prevention guidelines to prevent cardiovascular disease. This implicates that we need a more stratified follow-up program for women after a pregnancy with PE, which may facilitate treatment decisions for elevated blood pressure in a more timely manner than is currently done.
The first step in the follow-up of these women is adequate lifestyle advice and support provided by primary care physicians and dieticians. In addition, exercise training may improve physical condition [50]. Patient awareness of normal levels of CVD risk factors, especially blood pressure levels, is correlated with an increase in physical activity and improvements in diet. However, most women do not know their numbers and are not aware of normal thresholds [51]. Patient empowerment in lifestyle management and knowledge on normal values of the traditional risk factors will improve their awareness and motivation [52]. Self-measurement of blood pressure using eHealth facilities may be helpful to detect elevated blood pressure on time for adequate treatment.
Conclusion
Obstetric history can provide useful information about cardiovascular disease risk in women after their reproductive life, especially if they had a pregnancy complicated by preeclampsia. Evidence suggests that screening for cardiovascular risk factors, such as hypertension, to prevent cardiovascular disease should start soon after pregnancy. Early detection of hypertension could optimize prevention and treatment strategies and mitigate CVD risk. For high-risk women, preeclampsia may be considered a first cardiovascular event that requires secondary prevention guidelines.
Future investigations should focus on optimal screening methods for individual cardiovascular risk among women with preeclamptic pregnancies.
Abbreviations
- ACC :
-
American College of Cardiology
- ACS :
-
acute coronary syndrome
- AHA :
-
American Heart Association
- APRs :
-
acute-phase responses
- ASCVD :
-
atherosclerotic cardiovascular disease
- CAC :
-
coronary artery calcium
- CIMT :
-
carotid intima-media thickness
- CRP :
-
C-reactive protein
- CVD :
-
cardiovascular disease
- ESC :
-
European Society of Cardiology
- HDP :
-
hypertensive disorders of pregnancy
- HF :
-
heart failure
- IFN-γ :
-
interferon γ
- IL :
-
interleukin
- ISSHP :
-
International Society for the Study of Hypertension
- MI :
-
myocardial infarction
- NOS :
-
nitric oxide synthase
- PE :
-
preeclampsia
- TNF-α :
-
tumor necrosis factor α
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. https://doi.org/10.1136/bmj.39335.385301.BE.
Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. https://doi.org/10.1007/s10654-013-9762-6.
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. https://doi.org/10.1016/j.ahj.2008.06.042.
Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). https://doi.org/10.1161/circoutcomes.116.003497.
Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–23. https://doi.org/10.1016/j.jacc.2011.02.005.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81. https://doi.org/10.1093/eurheartj/ehw106.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2017. https://doi.org/10.1161/hyp.0000000000000066.
Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387(10022):999–1011. https://doi.org/10.1016/S0140-6736(15)00070-7.
Edstedt Bonamy A-K, Parikh NI. Predicting women’s future cardiovascular health from pregnancy complications. Curr Cardiovasc Risk Rep. 2013;7(3):173–82. https://doi.org/10.1007/s12170-013-0314-0.
Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertension. 2014;4(2):97–104. https://doi.org/10.1016/j.preghy.2014.02.001.
van Esch JJA, van Heijst AF, de Haan AFJ, van der Heijden OWH. Early-onset preeclampsia is associated with perinatal mortality and severe neonatal morbidity. J Matern Fetal Neonatal Med. 2017;30(23):2789–94. https://doi.org/10.1080/14767058.2016.1263295.
Schutte J, Steegers E, Schuitemaker N, Santema J, de Boer K, Pel M, et al. Rise in maternal mortality in the Netherlands. BJOG Int J Obstet Gynaecol. 2010;117(4):399–406. https://doi.org/10.1111/j.1471-0528.2009.02382.x.
American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. https://doi.org/10.1097/01.AOG.0000437382.03963.88.
• Arnaout R, Nah G, Marcus G, Tseng Z, Foster E, Harris IS, et al. Pregnancy complications and premature cardiovascular events among 1.6 million California pregnancies. Open Heart. 2019;6(1):e000927. https://doi.org/10.1136/openhrt-2018-000927 Arnaout et al. demonstrated in a retrospective cohort study in over 1.6 million women that HDP can predict future myocardial infarction, heart failure, and stroke. The authors showed that hypertensive disorders of pregnancy were associated with a 1.4-fold to 7.6-fold higher risk of MI, HF, and stroke. These findings support the close monitoring of women with HDP for the prevention of CVD events.
• Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. 2018;72(11):1252–63. https://doi.org/10.1016/j.jacc.2018.05.077 Stuart et al. determined whether the inclusion of HDP in an established CVD risk score improves the prediction of CVD events in women aged 40 years or older. The authors showed that the addition of HDP to the risk score did not improve the prediction of CVD events, but HDP and parity were independently associated with CVD in women aged 40 to 49 years. This result suggests that HDP can contribute to risk prediction and improve the identification of high-risk women to prevent the development of CVD risk factors.
Dam V, Onland-Moret NC, Verschuren WMM, Boer JMA, Benschop L, Franx A, et al. Cardiovascular risk model performance in women with and without hypertensive disorders of pregnancy. Heart. 2019;105(4):330–6. https://doi.org/10.1136/heartjnl-2018-313439.
Timpka S, Fraser A, Schyman T, Stuart JJ, Asvold BO, Mogren I, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol. 2018;33(10):1003–10. https://doi.org/10.1007/s10654-018-0429-1.
Breetveld NM, Ghossein-Doha C, van Kuijk S, van Dijk AP, van der Vlugt MJ, Heidema WM, et al. Cardiovascular disease risk is only elevated in hypertensive, formerly preeclamptic women. BJOG. 2015;122(8):1092–100. https://doi.org/10.1111/1471-0528.13057.
•• Zoet GA, Benschop L, Boersma E, Budde RPJ, Fauser BCJM, Graaf Y, et al. Prevalence of subclinical coronary artery disease assessed by coronary computed tomography angiography in 45- to 55-year-old women with a history of preeclampsia. Circulation. 2018;137(8):877–9. https://doi.org/10.1161/CIRCULATIONAHA.117.032695 Zoet et al. compared the prevalence of coronary artery atherosclerosis of asymptomatic women who have a history of preeclampsia with women who have had a normotensive pregnancy. The authors showed that preeclampsia is associated with a twofold higher risk to have identifiable coronary atherosclerosis. Furthermore, women with a history of preeclampsia had a higher prevalence of hypertension and metabolic syndrome. The authors demonstrated that preeclampsia is associated with coronary artery atherosclerosis at age 45 to 55 years, which precedes the development of subclinical ischemic heart disease.
Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39(41):3727–35. https://doi.org/10.1093/eurheartj/ehy534.
Lawes CMM, Hoorn SV, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–8. https://doi.org/10.1016/S0140-6736(08)60655-8.
Patel SA. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med. 2015;163(4):245–53.
Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA. 2011;305(9):913–22. https://doi.org/10.1001/jama.2011.250.
Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM). Eur J Prev Cardiol. 2012;19(5):1138–44. https://doi.org/10.1177/1741826711421079.
Groenhof TKJ, van Rijn BB, Franx A, Roeters van Lennep JE, Bots ML, Lely AT. Preventing cardiovascular disease after hypertensive disorders of pregnancy: searching for the how and when. Eur J Prev Cardiol. 2017;24(16):1735–45. https://doi.org/10.1177/2047487317730472.
Groenhof TKJ, Zoet GA, Franx A, Gansevoort RT, Bots ML, Groen H, et al. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73(1):171–8. https://doi.org/10.1161/hypertensionaha.118.11726.
Bokslag A, Teunissen PW, Franssen C, van Kesteren F, Kamp O, Ganzevoort W, et al. Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Obstet Gynecol. 2017;216(5):523 e1–7. https://doi.org/10.1016/j.ajog.2017.02.015.
Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, et al. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. 2015;66(6):1116–22. https://doi.org/10.1161/HYPERTENSIONAHA.115.06005.
Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169(4):224–32. https://doi.org/10.7326/m17-2740.
Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. https://doi.org/10.1136/bmj.j3078.
Veerbeek JH, Hermes W, Breimer AY, van Rijn BB, Koenen SV, Mol BW, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65(3):600–6. https://doi.org/10.1161/HYPERTENSIONAHA.114.04850.
Benschop L, Duvekot JJ, Versmissen J, van Broekhoven V, Steegers EAP, Roeters van Lennep JE. Blood pressure profile 1 year after severe preeclampsia. Hypertension. 2018;71:491–8. https://doi.org/10.1161/hypertensionaha.117.10338.
Cho GJ, Kim HY, Park JH, Ahn KH, Hong SC, Kim HJ, et al. Prepregnancy factors are associated with development of hypertension later in life in women with pre-eclampsia. J Women’s Health (Larchmt). 2018. https://doi.org/10.1089/jwh.2018.7165.
Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122(6):579–84. https://doi.org/10.1161/circulationaha.110.943407.
Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70. https://doi.org/10.1093/epirev/mxt006.
Egeland GM, Skurtveit S, Staff AC, Eide GE, Daltveit AK, Klungsoyr K, et al. Pregnancy-related risk factors are associated with a significant burden of treated hypertension within 10 years of delivery: findings from a population-based Norwegian cohort. J Am Heart Assoc. 2018;7(10). https://doi.org/10.1161/jaha.117.008318.
Cornelius DC. Preeclampsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord. 2018;11: 1179545X17752325 https://doi.org/10.1177/1179545X17752325.
Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clinical science (London, England : 1979). 2016;130(6):409–19. https://doi.org/10.1042/cs20150702.
Girouard J, Giguère Y, Moutquin J-M, Forest J-C. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension. 2007;49(5):1056–62. https://doi.org/10.1161/HYPERTENSIONAHA.107.087528.
Drost JT, Maas AH, Holewijn S, Joosten LA, van Eyck J, van der Schouw YT, et al. Novel cardiovascular biomarkers in women with a history of early preeclampsia. Atherosclerosis. 2014;237(1):117–22. https://doi.org/10.1016/j.atherosclerosis.2014.09.009.
van Rijn BB, Franx A, Steegers EAP, de Groot CJM, Bertina RM, Pasterkamp G, et al. Maternal TLR4 and NOD2 gene variants, pro-inflammatory phenotype and susceptibility to early-onset preeclampsia and HELLP syndrome. PLoS One. 2008;3(4):e1865–e. https://doi.org/10.1371/journal.pone.0001865.
BBv R, Bruinse HW, Veerbeek JH, Uiterweer EDP, Koenen SV, JGvd B, et al. Postpartum circulating markers of inflammation and the systemic acute-phase response after early-onset preeclampsia. Hypertension. 2016;67(2):404–14. https://doi.org/10.1161/HYPERTENSIONAHA.115.06455.
Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol. 2017;8:1058. https://doi.org/10.3389/fimmu.2017.01058.
Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart J-C, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109(23_suppl_1):III-33–I-8. https://doi.org/10.1161/01.CIR.0000131516.65699.ba.
Kablak-Ziembicka A, Przewlocki T, Tracz W, Pieniazek P, Musialek P, Sokolowski A, et al. Carotid intima-media thickness in pre- and postmenopausal women with suspected coronary artery disease. Heart Vessel. 2008;23(5):295–300. https://doi.org/10.1007/s00380-008-1044-y.
Milic NM, Milin-Lazovic J, Weissgerber TL, Trajkovic G, White WM, Garovic VD. Preclinical atherosclerosis at the time of pre-eclamptic pregnancy and up to 10 years postpartum: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49(1):110–5. https://doi.org/10.1002/uog.17367.
Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of vascular dysfunction after hypertensive disorders of pregnancy. Hypertension 2016;68(6):1447–1458. doi:https://doi.org/10.1161/HYPERTENSIONAHA.116.07907.
de Jager SCA, Meeuwsen JAL, van Pijpen FM, Zoet GA, Barendrecht AD, Franx A, et al. Preeclampsia and coronary plaque erosion: manifestations of endothelial dysfunction resulting in cardiovascular events in women. Eur J Pharmacol. 2017. http://www.sciencedirect.com/science/article/pii/S0014299917305903;816:129–37.
Grand'Maison S, Pilote L, Schlosser K, Stewart DJ, Okano M, Dayan N. Clinical features and outcomes of acute coronary syndrome in women with previous pregnancy complications. Can J Cardiol. 2017;33(12):1683–92. https://doi.org/10.1016/j.cjca.2017.08.025.
Scholten RR, Thijssen DJ, Lotgering FK, Hopman MT, Spaanderman ME. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol. 2014;211(5):516.e1–e11. https://doi.org/10.1016/j.ajog.2014.04.025.
Mosca L, Mochari H, Christian A, Berra K, Taubert K, Mills T, et al. National study of women’s awareness, preventive action, and barriers to cardiovascular health circulation. 2006;113(4):525–34. https://doi.org/10.1161/CIRCULATIONAHA.105.588103.
Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(11):1278–93. https://doi.org/10.1016/j.jacc.2018.07.008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hella E.C. Muijsers, Nel Roeleveld, Olivier W.H. van der Heijden, and Angela H.E.M. Maas declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Search Strategy
We searched PubMed with the following terms: “preeclampsia,” “pre-eclampsia,” “cardiovascular disease,” and “cardiovascular disease risk factors.” We focused on research published in the last 5 years, but made an exception for older key publications.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Women and Heart Disease
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Muijsers, H.E.C., Roeleveld, N., van der Heijden, O.W.H. et al. Consider Preeclampsia as a First Cardiovascular Event. Curr Cardiovasc Risk Rep 13, 21 (2019). https://doi.org/10.1007/s12170-019-0614-0
Published:
DOI: https://doi.org/10.1007/s12170-019-0614-0