Abstract
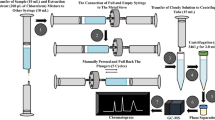
The occurrence of antibiotic residues in milk constitutes a potential risk to the health of consumers. The present study describes the optimisation and validation of a high-performance liquid chromatographic (HPLC) method for the simultaneous determination of sulphadiazine (SDZ), sulphamethoxazole (SMX), oxytetracycline (OTC), doxycycline (DOX), tetracycline (TC), enrofloxacin (ENRO) and chloramphenicol (CLP) residues in bovine milk using colchicine (COL) as internal standard. The determination of these antimicrobials was carried out on C18 analytical column using high-performance liquid chromatographic-diode array detection (HPLC-DAD). The extraction method involving deproteinisation of the milk sample followed by a solid-phase extraction (SPE) clean-up of antibiotic residues has been developed. The method was validated according to the European Commission Decision 2002/657/EC and applied for the analysis of antibiotic residues in 21 raw milk samples collected from Ludhiana, Punjab, India. The recoveries for the studied antibiotics ranged from 83.3–111.8% with relative standard deviations between 3.5 and 16.2%. The limits of quantification for these antibiotics, with the exception of chloramphenicol, were below the maximum residue limits (MRLs), making the method suitable for performing routine analysis.



Similar content being viewed by others
References
Ahlberg S, Korhonen H, Lindfors E, Kangethe E (2006) Analysis of antibiotic residues in milk from smallholder farms in Kenya. Afri J Dairy Farm Milk Prod 3:152–158
Andersen WC, Roybal JE, Gonzales SA, Turnipseed SB, Pfenning AP, Kuck LR (2005) Determination of tetracycline residues in shrimp and whole milk using liquid chromatography with ultraviolet detection and residue confirmation by mass spectrometry. Anal Chim Acta 529:145–150
Andrew SM, Moyes KM, Borm AA, Fox LK, Leslie KE, Hogan JS et al (2009) Factors associated with the risk of antibiotic residues and intramammary pathogen presence in milk from heifers administered prepartum intramammary antibiotic therapy. Vet Microbiol 134:150–156
Bilandzic N, Kolanovic BS, Varenina I, Jurkovic Z (2011) Concentrations of veterinary drug residues in milk from individual farms in Croatia. Mljekarstvo 61:260–267
Brtio RB, Junqueira RG (2006) Determination of beta-lactam residues in milk by high performance liquid chromatography. Braz Arch Biol Technol 49:41–46
Camara M, Gallego-Pico A, Garcinuno RM, Fernandez-Hernando P, Durand-Alegría JS, Sanchez PJ (2003) An HPLC-DAD method for the simultaneous determination of nine β-lactam antibiotics in ewe milk. Food Chem 141:829–834
Cinquina AL, Longo F, Anastasi G, Giannetti L, Cozzani R (2003) Validation of a high performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle. J Chromatogr A 987:227–233
Codex alimentarius commission (2017) Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. Updated at the 40th session of Codex alimentarius commission, July 2017
European Commission (2002) Comission Regulation of 2002/657/EC, 12 August. Implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Communities, L 221/8-L221/36. 2002
European Commission (2010) Commission Regulation of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin, 37/2010/EU. In: Off J, L 15, 20/01/2010, pp 1–72. 2010. Available at https://ec.europa.eu/health//sites/health/files/files/mrl/mrl_20101212_consol.p f Accessed February 3, 2018
Fritz JW, Zuo Y (2007) Simultaneous determination of tetracycline, oxytetracycline and 4-epitetracycline in milk by high performance liquid chromatography. Food Chem 105:1297–1301
Furusawa N, Kishida K (2001) High-performance liquid chromatographic procedure for routine residue monitoring of seven sulfonamides in milk. Fresenius J Anal Chem 371:1031–1033
Guidi LR, Silva LHM, Fernandes C, Engeseth NJ, Gloria MBA (2015) LC-MS/MS determination of chloramphenicol in food of animal origin in Brazil. Sci Chromatogr 7:287–295
Hassouan M, Ballesteros O, Vílchez J, Zafra A, Navalon A (2007) Simple multiresidue determination of fluoroquinolones in bovine milk by liquid chromatography with fluorescence detection. Anal Lett 40:779–791
Huelamo MM, Gmez EJ, Hermo MP, Barron D, Barbosa J (2009) Determination of penicillins in milk using LC-UV, LC-MS and LC-MS/MS. J Sep Sci 32:2385–2393
Jahed-Khanik GR (2007) Chemical contaminants in milk and public health concerns: a review. Int J Dairy Sci 2:104–115
Jank L, Martins MT, Arsand JB, Motta TMC, Feijo TC, Castilhos TS, Hoff RB, Barreto F, Pizzolato TM (2017) Liquid chromatography-tandem mass spectrophotometry multiclass method for 46 antibiotics residues in milk and meat: development and validation. Food Anal Methods 10:2152–2164
Kebede G, Zenebe T, Disassa H, Tolosa T (2014) Review on detection of antimicrobial residues in raw bulk milk in dairy farms. Afr J Basic Appl Sci 6:87–97
Kivrak I, Kivrak S, Harmandar M (2016) Development of a rapid method for the determination of antibiotic residues in honey using UPLC-ESI-MS/MS. Food Sci Technol 36:90–96
Liu ZQ, Lu SX, Zhao CH, Ding K, Cao ZZ, Zhan JH, Ma C, Liu JT, Xi RM (2009) Preparation of anti-danofloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residue in chicken liver. J Sci Food Agric 89:1115–1121
Long AR, Hsieh Lily C, Bello AC, Malbrough MS, Short CR, Barker SA (1999) Method for the isolation and liquid chromatographic determination of chloramphenicol in milk. J Agric Food Chem 38:427–429
Mamani MCV, Reyes FGR, Rath S (2009) Multiresidue determination of tetracyclines, sulphonamides and chloramphenicol in bovine milk using HPLC-DAD. Food Chem 117:545–552
Marazuela MD, Moreno-Bondi MC (2004) Multi-residue determination of fluoroquinolones in milk by column liquid chromatography with fluorescence and ultraviolet absorbance detection. J Chromatogr A 1034:25–32
Martins MT, Barreto F, Hoff RB, Jank L, Arsand JB, FeijO TC, Schapoval EES (2015) Determination of quinolones and fluoroquinolones, tetracyclines and sulfonamides in bovine, swine and poultry liver using LC-MS/MS. Food Addit Contam Part A 32:333–341
Moudgil P, Bedi JS, Moudgil AD, Gill JPS, Aulakh RS (2017) Emerging issue of antibiotic resistance from food producing animals in India: perspective and legal framework. Food Rev Int 34:447–462. https://doi.org/10.1080/87559129.2017.1326934
Nisha AR (2008) Antibiotics residues: a global health hazard. Vet World 112:375–377
Pikkemaat MG (2009) Microbial screening methods for detection of antibiotic residues in slaughter animals. Anal Chim Acta 395:895–905
Raz SR, Bremer MG, Haasnoot W, Norde W (2009) Label-free and multiplex detection of antibiotic residues in milk using imaging surface plasmon resonance-based immunosensor. Anal Chem 81:7743–7749
Samanidou VF, Nikolaidou KI, Papadoyannis IN (2007) Development and validation of an HPLC confirmatory method for the determination of seven tetracycline antibiotics residues in milk according to the European Union Decision 2002/657/EC. J Sep Sci 30:2430–2439
Samanidou VF, Tolika EP, Papadoyannis IN (2008) Development and validation of an HPLC confirmatory method for the residue analysis of four sulphonamides in cow’s milk according to the European Union Decision 2002/657/EC. J Liq Chromatogr Relat Technol 31:1358–1372
Toaldo IM, Gamba GZ, Picinin LA, Rubensam G, Hoff R, Luiz MB (2012) Multiclass analysis of antibacterial residues in milk using RP-liquid chromatography with photodiode array and fluorescence detection and tandem mass spectrometer confirmation. Talanta 99:616–624
Van Rhijn JA, Lasaroms JJP, Berendsen BJA, Brinkman UAT (2002) Liquid chromatographic–tandem mass spectrometric determination of selected sulphonamides in milk. J Chromatogr A 960:121–133
Wang J, Leung D, Chow W, Chang J, Wong JW (2015) Development and validation of a multiclass method for analysis of veterinary drug residues in milk using ultrahigh performance liquid chromatography electrospray ionization quadrupole orbitrap mass spectrometry. J Agric Food Chem 63:9175–9187
Wu YL, Li C, Liu YJ, Shen JZ (2007) Validation method for the determination of sulfonamide residues in bovine milk by HPLC. Chromatographia 66:197–199
Zhao F, Zhang X, Gan Y (2004) Determination of tetracyclines in ovine milk by high-performance liquid chromatography with a coulometric electrode array system. J Chromatogr A 1055:109–114
Acknowledgements
Authors are thankful to “Rashtriya Krishi Vikas Yojana” (RKVY), Government of India, for providing funds for undertaking the study through project entitled “Studies on current scenario of antibiotic residues in food of animal origin in Punjab and prevention of antibiotic residue risks”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pallavi Moudgil declares that she has no conflict of interest. Jasbir Singh Bedi declares that he has no conflict of interest. Rabinder Singh Aulakh declares that he has no conflict of interest. Jatinder Paul Singh Gill declares that he has no conflict of interest. Amit Kumar declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Moudgil, P., Bedi, J.S., Aulakh, R.S. et al. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Methods 12, 338–346 (2019). https://doi.org/10.1007/s12161-018-1365-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1365-0