Abstract
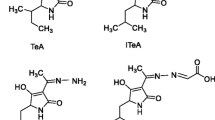
Alternariol (AOH) is one of the major mycotoxins produced by various species of Alternaria fungi. Natural occurrences of AOH have been reported in various foods, including fruits; processed fruit products such as apple juice, tomato products; wheat and other grains; oilseeds and products thereof, such as sunflower seeds, oilseed rape meal, and flax seed/linseed; and pecans. In this study, AOH-specific polyclonal antibodies were generated and developed an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for monitoring AOH in bread and bran samples. The assay was very sensitive with a limit of detection (LOD) of 2.4 ± 0.6 ng/g and a half maximal inhibitory concentration (IC50) of 15.2 ± 2.6 ng/g in bread and a LOD of 8.4 ± 1.2 ng/g and IC50 of 52.8 ± 10.8 ng/g in bran extract. The assay was very specific to AOH and showed no cross-reactivity to alternariol monomethyl ether, altertoxin, altenuene, tentoxin, or tenuazonic acid. The effect of organic solvents on the assay was tested. The ELISA system tolerated methanol and acetonitrile as co-solvents at up to 5% content without significant loss of IC50 value. Recoveries in all cases were greater than 75%, and the results using this method were comparable to those obtained from mass spectrometry methods. We conclude that this method is suitable for rapid detection of AOH in bread and bran samples, without expensive analytical equipment or time-consuming sample preparation.


Similar content being viewed by others
References
Ackermann Y, Curtui V, Dietrich R, Gross M, Latif H, Martlbauer E, Usleber E (2011) Widespread occurrence of low levels of alternariol in apple and tomato products, as determined by comparative immunochemical assessment using monoclonal and polyclonal antibodies. J Agric Food Chem 59(12):6360–6368. https://doi.org/10.1021/jf201516f
Bauer JI, Gross M, Gottschalk C, Usleber E (2016) Investigations on the occurrence of mycotoxins in beer. Food Control 63:135–139. https://doi.org/10.1016/j.foodcont.2015.11.040
Brugger EM, Wagner J, Schumacher DM, Koch K, Podlech J, Metzler M, Lehmann L (2006) Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol Lett 164(3):221–230. https://doi.org/10.1016/j.toxlet.2006.01.001
Burkin AA, Kononenko GP (2011) Enzyme immunoassay of alternariol for the assessment of risk of agricultural products contamination. Appl Biochem Microbiol 47(1):72–76. https://doi.org/10.1134/S0003683811010030
EFSA (2011) Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J 9:2407
Fleck SC, Burkhardt B, Pfeiffer E, Metzler M (2012) Alternaria toxins: altertoxin II is a much stronger mutagen and DNA strand breaking mycotoxin than alternariol and its methyl ether in cultured mammalian cells. Toxicol Lett 214(1):27–32. https://doi.org/10.1016/j.toxlet.2012.08.003
Hickert S, Gerding J, Ncube E, Hübner F, Flett B, Cramer B, Humpf HU (2015) A new approach using micro HPLC-MS/MS for multi-mycotoxin analysis in maize samples. Mycotoxin Research 31(2):109–115. https://doi.org/10.1007/s12550-015-0221-y
Hickert S, Bergmann M, Ersen S, Cramer B, Humpf HU (2016) Survey of Alternaria toxin contamination in food from the German market, using a rapid HPLC-MS/MS approach. Mycotoxin Research 32(1):7–18. https://doi.org/10.1007/s12550-015-0233-7
Juan C, Oueslati S, Mañes J (2016) Evaluation of Alternaria mycotoxins in strawberries: quantification and storage condition Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment 33:861–868, 5, DOI: https://doi.org/10.1080/19440049.2016.1177375
Lehmann L, Wagner J, Metzler M (2006) Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem Toxicol 44(3):398–408. https://doi.org/10.1016/j.fct.2005.08.013
Liu GT et al. (1991) Relationships between Alternaria alternata and oesophageal cancer. IARC Sci Publ:258–262
Liu GT, Qian YZ, Zhang P, Dong WH, Qi YM, Guo HT (1992) Etiological role of Alternaria alternata in human esophageal cancer. Chin Med J 105(5):394–400
Logrieco A, Moretti A, Solfrizzo M (2009) Alternaria toxins and plant diseases: an overview of origin, occurrence and risks. World Mycotoxin J 2(2):129–140. https://doi.org/10.3920/WMJ2009.1145
López P, Venema D, de Rick T, de Kok A, Scholten JM, Mol HGJ, de Nijs M (2016) Occurrence of Alternaria toxins in food products in The Netherlands. Food Control 60:196–204. https://doi.org/10.1016/j.foodcont.2015.07.032
Noser J, Schneider P, Rother M, Schmutz H (2011) Determination of six Alternaria toxins with UPLC-MS/MS and their occurrence in tomatoes and tomato products from the Swiss market. Mycotoxin Research 27(4):265–271. https://doi.org/10.1007/s12550-011-0103-x
Ostry V (2008) Alternaria mycotoxins: an overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J 1(2):175–188. https://doi.org/10.3920/WMJ2008.x013
Prelle A, Spadaro D, Garibaldi A, Gullino ML (2013) A new method for detection of five Alternaria toxins in food matrices based on LC-APCI-MS. Food Chem 140(1-2):161–167. https://doi.org/10.1016/j.foodchem.2012.12.065
Schrader TJ, Cherry W, Soper K, Langlois I (2006) Further examination of the effects of nitrosylation on Alternaria alternata mycotoxin mutagenicity in vitro. Mutation Research - Genetic Toxicology and Environmental Mutagenesis 606(1-2):61–71. https://doi.org/10.1016/j.mrgentox.2006.02.008
Scott PM (2001) Analysis of agricultural commodities and foods for Alternaria mycotoxins. J AOAC Int 84(6):1809–1817
Scott PM, Kanhere SR (2001) Stability of Alternaria toxins in fruit juices and wine. Mycotoxin Research 17(1):9–14. https://doi.org/10.1007/BF02946112
Scott PM, Weber D, Kanhere SR (1997) Gas chromatography-mass spectrometry of Alternaria mycotoxin. J Chromatogr A 765(2):255–263. https://doi.org/10.1016/S0021-9673(96)00917-X
Scott PM, Lawrence GA, Lau BPY (2006) Analysis of wines, grape juices and cranberry juices for Alternaria toxins. Mycotoxin Research 22(2):142–147. https://doi.org/10.1007/BF02956778
Scott PM, Zhao W, Feng S, Lau BPY (2012) Alternaria toxins alternariol and alternariol monomethyl ether in grain foods in Canada. Mycotoxin Research:1–6
Singh G, Brady B, Koerner T, Becalski A, Zhao T, Feng S, Godefroy SB, Huet AC, Delahaut P (2014) Development of a highly sensitive competitive indirect enzyme-linked immunosorbent assay for detection of acrylamide in foods and water. Food Anal Methods 7(6):1298–1304. https://doi.org/10.1007/s12161-013-9749-7
Solfrizzo M (2017) Recent advances on Alternaria mycotoxins Current Opinion in Food Science 17:57–61
Zhao K, Shao B, Yang D, Li F, Zhu J (2015) Natural occurrence of Alternaria toxins in wheat-based products and their dietary exposure in China. PLoS One 10
Zwickel T, Klaffke H, Richards K, Rychlik M (2016) Development of a high performance liquid chromatography tandem mass spectrometry based analysis for the simultaneous quantification of various Alternaria toxins in wine, vegetable juices and fruit juices. J Chromatogr A 1455:74–85. https://doi.org/10.1016/j.chroma.2016.04.066
Acknowledgements
The authors thank Wendy Zhao, FRD Bureau of Chemical Safety, Health Canada, for providing the commercial samples which were analyzed by LC-MS/MS method in our division.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gurmit Singh declares that he has no conflict of interest. Ligia Velasquez declares that she has no conflict of interest. Beth Brady declares that she has no conflict of interest. Terry Koerner declares that he has no conflict of interest. Anne-Catherine Huet declares that she has no conflict of interest. Philippe Delahaut declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human subjects. All animal experiments that described in the present study were performed in CER Groupe, Belgium, following all institutional and national guidelines for the care and use of laboratory animals.
Informed Consent
Not applicable
Rights and permissions
About this article
Cite this article
Singh, G., Velasquez, L., Brady, B. et al. Development of an Indirect Competitive ELISA for Analysis of Alternariol in Bread and Bran Samples. Food Anal. Methods 11, 1444–1450 (2018). https://doi.org/10.1007/s12161-017-1126-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1126-5