Abstract
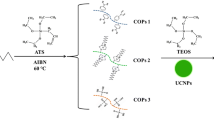
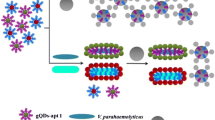
Rare earth-doped upconversion nanoparticles (UCNPs) have promising potential in biodetection due to their peculiar frequency upconverting capabilities and high detection sensitivity. Here, we report a novel dual-color UCNP-based bacterium-sensing biosensor for simultaneously Escherichia coli and Staphylococcus aureus detection using UCNP as a fluorescence marker and conjugated with antibodies as the specific molecular recognition probe. Dual-color UCNPs were fabricated via varying lanthanide dopants to acquire the well-separated emission peaks. When E. coli and S. aureus were added into the reaction system, the recognition probes would capture the target bacteria through the specific binding of antibody. Then, the fluorescence intensities decreased (∆I = I o -I) were observed to increase linearly with the rising concentration of the E. coli (664 nm) and S. aureus (806 nm) from 47 to 47 × 106 cfu mL−1 (R 2 = 0.98) and 64 to 64 × 106 cfu mL−1 (R 2 = 0.97), respectively, resulting in the relatively low limit of 13 and 15 cfu mL−1 for E. coli and S. aureus. Furthermore, this UCNP-based bacterium-sensing biosensor could also be successfully applied to simultaneously detect E. coli and S. aureus in adulterated meat and milk samples.





Similar content being viewed by others
References
Ang LY, Lim ME, Ong LC, Zhang Y (2011) Applications of upconversion nanoparticles in imaging, detection and therapy. Nanomedicine 6(7):1273–1288
Bottrill M, Green M (2011) Some aspects of quantum dot toxicity. Chem Commun 47(25):7039–7050
Cheng L, Wang C, Liu Z (2013) Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy. Nanoscale 5(1):23–37
Chung HJ, Castro CM, Im H, Lee H, Weissleder R (2013) A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nat Nanotechnol 8(5):369–375
Deng M, Wang L (2014) Unexpected luminescence enhancement of upconverting nanocrystals by cation exchange with well retained small particle size. Nano Res 7(5):782–793
Gu Z, Yan L, Tian G, Li S, Chai Z, Zhao Y (2013) Recent advances in design and fabrication of upconversion nanoparticles and their safe theranostic applications. Adv Mater 25(28):3758–3779
Haase M, Schäfer H (2011) Upconverting nanoparticles. Angew Chem Int Ed 50(26):5808–5829
Hao S, Chen G, Yang C (2013) Sensing using rare-earth-doped upconversion nanoparticles. Theranostics 3(5):331–345
Huang P, Lin J, Wang S, Zhou Z, Li Z, Wang Z, Zhang C, Yue X, Niu G, Yang M (2013) Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials 34(19):4643–4654
Iqbal SS, Mayo MW, Bruno JG, Bronk BV, Batt CA, Chambers JP (2000) A review of molecular recognition technologies for detection of biological threat agents. Biosens Bioelectron 15(11):549–578
Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Christa L, Walker F, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ (2015) World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. Plos Medicine 12(12):22–27
Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G (2010) Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6(1):e1000715
Liu Q, Sun Y, Yang T, Feng W, Li C, Li F (2011) Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in vivo. J Am Chem Soc 133(43):17122–17125
Liu Y, Chen M, Cao TY, Sun Y, Li C Y, Liu Q, Yang TS, Yao LM, Feng W, Li F Y* (2013) A cyanine-modified nanosystem for upconversion luminescence bioimaging of methylmercury. J Am Chem Soc 135(26):9869–9876
Luo J, Liu X, Tian Q, Yue W, Zeng J, Chen G, Cai X (2009) Disposable bioluminescence-based biosensor for detection of bacterial count in food. Anal Biochem 394(1):1–6
Miskinyte M, Sousa A, Ramiro RS, de Sousa JAM, Kotlinowski J, Caramalho I, Magalhães S, Soares MP, Gordo I (2013) The genetic basis of Escherichia coli pathoadaptation to macrophages. PLoS Pathog 9(12):e1003802
Niu W, Wu S, Zhang S (2010) A facile and general approach for the multicolor tuning of lanthanide-ion doped NaYF4 upconversion nanoparticles within a fixed composition. J Mater Chem 20(41):9113–9117
Ong LC, Ang LY, Alonso S, Zhang Y (2014) Bacterial imaging with photostable upconversion fluorescent nanoparticles. Biomaterials 35(9):2987–2998
Pan W, Zhao J, Chen Q (2015) Fabricating upconversion fluorescent probes for rapidly sensing foodborne pathogens. J Agric Food Chem 63(36):8068–8074
Roda A, Mirasoli M, Roda B, Bonvicini F, Colliva C, Reschiglian P (2012) Recent developments in rapid multiplexed bioanalytical methods for foodborne pathogenic bacteria detection. Microchim Acta 178(1–2):7–28
Shan S, Lai W, Xiong Y, Wei H, Xu H (2015) Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J Agric Food Chem 63(3):745–753
Sharma H, Mutharasan R (2013) Review of biosensors for foodborne pathogens and toxins. Sensors Actuators B Chem 183:535–549
Smith AM, Mancini MC, Nie S (2009) Second window for in vivo imaging. Nat Nanotechnol 4(11):710
Song E, Yu M, Wang Y, Hu W, Cheng D, Swihart MT, Song Y (2015) Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens Bioelectron 72:320–325
Tram K, Kanda P, Salena BJ, Huan S, Li Y (2014) Translating bacterial detection by DNAzymes into a litmus test. Angew Chem Int Ed 53(47):12799–12802
Velusamy V (2012) Design, development and characterization of a handheld electrochemical analyzer system: in the perspective of DNA biosensors for foodbourne pathogen detection. University of Limerick 46:17–22
Wang F, Banerjee D, Liu Y, Chen X, Liu X (2010a) Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 135(8):1839–1854
Wang F, Deng R, Wang J, Wang Q, Han Y, Zhu H, Chen X, Liu X (2011) Tuning upconversion through energy migration in core–shell nanoparticles. Nat Mater 10(12):968–973
Wang F, Han Y, Lim CS, Lu Y, Wang J, Xu J, Chen H, Zhang C, Hong M, Liu X (2010b) Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 463(7284):1061–1065
Wu S, Duan N, Shi Z, Fang C, Wang Z (2014) Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal Chem 86(6):3100–3107
Wu W, Zhao S, Mao Y, Fang Z, Lu X, Zeng L (2015) A sensitive lateral flow biosensor for Escherichia coli O157: H7 detection based on aptamer mediated strand displacement amplification. Anal Chim Acta 861:62–68
Yang ST, Cao L, Luo PG, Lu F, Wang X, Wang H, Meziani MJ, Liu Y, Qi G, Sun YP (2009) Carbon dots for optical imaging in vivo. J Am Chem Soc 131(32):11308–11309
Zhang H, Ma X, Liu Y, Duan N, Wu S, Wang Z, Xu B (2015) Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens Bioelectron 74:872–877
Acknowledgements
This work has been financially supported by the National Natural Science Foundation of China (31371770), Key R&D Program of Jiangsu Province (No. BE2015302), Postgraduate Innovative Program for Higher Education Institutions in Jiangsu Province (KYLX16_0913), the Natural Science Foundation of Jiangsu Province (Youth) (BK20150502), the China Postdoctoral Science Foundation (2015M571698), and the Advanced Talents Science Foundation of Jiangsu University (15JDG064).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study has no financial relationship with the organization that sponsored the research.
Conflict of Interest
Bin Zhang declares that he has no conflict of interest. Huanhuan Li declares that he has no conflict of interest. Wenxiu Pan declares that he has no conflict of interest. Quansheng Chen declares that he has no conflict of interest. Qin Ouyang declares that he has no conflict of interest. Jiewen Zhao declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Electronic supplementary material
ESM 1
(DOCX 3242 kb)
Rights and permissions
About this article
Cite this article
Zhang, B., Li, H., Pan, W. et al. Dual-Color Upconversion Nanoparticles (UCNPs)-Based Fluorescent Immunoassay Probes for Sensitive Sensing Foodborne Pathogens. Food Anal. Methods 10, 2036–2045 (2017). https://doi.org/10.1007/s12161-016-0758-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0758-1