Abstract
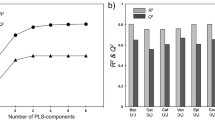
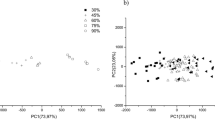
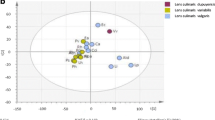
Nowadays, the traceability and origin authentification in foodstuff is of great interest for consumers and industries. It is proved that the chemical composition is linked to the geographical and varietal origin of food products. Cocoa, the main ingredient in chocolate, provides a lot of polyphenol compounds still present in the chocolate bars. By analyzing these chemical species, we aim to investigate the information about the country of origin of chocolate. Our method is based on an acetone/water liquid/liquid extraction coupled with a high-performance liquid chromatography–diode array detector–mass spectrometry analysis (HPLC-DAD-MS). Forty-seven chocolate samples of different varieties (Criollo, Trinitario, Nacional, and Forastero) from 12 countries within two continents were analyzed. Polyphenols such as catechin, epicatechin, and several of procyanidins’ polymers were identified. Principal component analysis (PCA) was performed on 21 variables: 20 polyphenols and the polyphenol total content. The results highlight that the polyphenolic profile allows to classify the chocolate samples according to geographical origins (Madagascar, Caribbean, different countries from South America and Africa) as well as their variety.







Similar content being viewed by others
References
Caligiani A, Cirlini M, Palla G, Ravaglia R, Arlorio M (2007) GC-MS detection of chiral markers in cocoa beans of different quality and geographic origin. Chirality 19:329–334
Cambrai A, Marcic C, Morville S, Sae Houer P, Bindler F, Marchioni E (2010) Differentiation of chocolates according to the cocoa’s geographical origin using chemometrics. J Agric Food Chem 58:1478–1483
Cooper KA, Campos-Giménez E, Jiménez Alvarez D, Nagy K, Donovan JL, Williamson G (2007) Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J Agric Food Chem 55:2841–2847
Di Mattia C, Martuscelli M, Sacchetti G, Beheydt B, Mastrocola D, Pittia P (2014) Effect of different conching processes on procyanidin content and antioxidant properties of chocolate. Food Res Int 63:367–372
Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH (1999) Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem 47:490–496
Hatano T, Miyatake H, Natsume M, Osakabe N, Takizawa T, Ito H, Yoshida T (2002) Proanthocyanidin glycosydes and related polyphenols from cocoa liquor and their antioxydant effects. Phytochemistry 59:749–758
Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH (2006) High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J Agric Food Chem 54(5):1571–1576
Kyi TM, Daud WRW, Mohamad AB, Samsudin MW, Kadhum AAH, Talib MZM (2005) The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans. Int J Food Sci Technol 40:323–331
Longobardi F, Ventrella A, Casiello G, Sacco D, Catucci L, Agostiano A, Kontominas MG (2012) Instrumental and multivariate statistical analyses for the characterization of the geographical origin of Apulian virgin olive oils. Food Chem 133:579–584
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Marseglia A, Acquotti D, Consonni R, Cagliani LR, Palla G, Cagliani A (2016) HR MAS 1H NMR and chemometrics as useful tool to assess the geographical origin of cocoa beans—comparison with HR 1H NMR. Food Res Int 85:273–281
Miller JN, Miller JC (2010) Statistics and chemometrics for analytical chemistry, 6th edn. Pearson Education Limited, Harlow, England
Natsume M, Osakabe N, Yamagishi M, Takizawa T, Nakamura T, Miyatake H, Hatano T, Yoshida T (2000) Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase LC. Biosci Biotechnol Biochem 64:2581–2587
Nazaruddin R, Seng LK, Hassan O, Said M (2006) Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma cacao) during fermentation. Ind Crop Prod 24:87–94
Niemenak N, Rohsius C, Elwers S, Omokolo Ndoumou D, Lieberei R (2006) Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. J Food Compos Anal 19:612–619
Ortega N, Romero MP, Macia A, Reguant J, Anglès N, Morello JR, Motilva MJ (2010) Comparative study of UPLC–MS/MS and HPLC–MS/MS to determine procyanidins and alkaloids in cocoa samples. J Food Compos Anal 23:298–305
Othman A, Ismail A, Abdul Ghani N, Adenan I (2007) Antioxidant capacity and phenolic content of cocoa beans. Food Chem 100:1523–1530
Profeta A, Balling R, Roosen J (2012) The relevance of origin information at the point of sale. Food Qual Prefer 26:1–11
Sall J, Creighton L, Lehman A (2005) JMP ® start statistics, a guide to statistics and data analysis using JMP ® and JMP IN ® software, 3rd edn. SAS Institute Inc./Thomson Brooks/Cole, Belmont
Saltini R, Akkerman R, Frosch S (2013) Optimizing chocolate production through traceability: a review of the influence of farming practices on cocoa bean quality. Food Control 29:167–187
Sanchez-Rabaneda F, Jauregui O, Casals I, Andres-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventos RM (2003) Liquid chromatographic / electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J Mass Spectrom 38(1):35–42
Schinella G, Mosca S, Cienfuegos-Jovellanos E, Ángeles Pasamar M, Muguerza B, Ramón D, Ríos JL (2010) Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res Int 43:1614–1623
Serra Bonvehi J, Ventura Coil F (1997) Evaluation of bitterness and astringency of polyphenolic compounds in cocoa powder. Food Chem 60(3):365–370
Stark T, Bareuther S, Hofmann T (2005) Sensory-guided decomposition of roasted cocoa nibs (Theobroma cacao) and structure determination of taste-active polyphenols. J Agric Food Chem 53:5407–5418
Tanaka T, Kondou K, Kouno I (2000) Oxidation and epimerisation of epigallocatechin in banana fruits. Phytochemistry 53:311–316
Wollgast J, Anklam E (2000) Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int 33:423–447
Wollgast J, Pallaroni L, Agazzi ME, Anklam E (2001) Analysis of procyanidins in chocolate by reversed-phase high-performance liquid chromatography with electrospray ionisation mass spectrometric and tandem mass spectrometric detection. J Chromatogr A 926:211–220
Żyżelewicz D, Krysiak W, Oracz J, Sosnowska D, Budryn G, Nebesny E (2016) The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res Int. doi:10.1016/j.foodres.2016.03.026
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Amandine Cambrai declares that she has no conflict of interest. Eric Marchioni declares that he has no conflict of interest. Diane Julien-David declares that she has no conflict of interest. Christophe Marcic declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Cambrai, A., Marchioni, E., Julien-David, D. et al. Discrimination of Cocoa Bean Origin by Chocolate Polyphenol Chromatographic Analysis and Chemometrics. Food Anal. Methods 10, 1991–2000 (2017). https://doi.org/10.1007/s12161-016-0744-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0744-7