Abstract
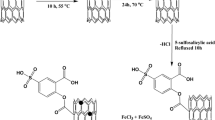
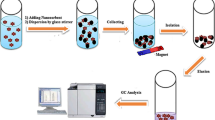
A novel functionalized magnetic multiwalled carbon nanotube composite was prepared and utilized as a nanosorbent for separation and preconcentration of Cr(III), Cd(II), Cu(II), Pb(II), and Ni(II) ions. The synthesized nanosorbent was characterized with various techniques. The parameters influencing the preconcentration efficiency were optimized through experimental design methodology by using Box-Behnken design method. Uptake time, pH of sample, and magnetic nanosorbent amount were evaluated in the sorption step as the main affecting factors, while four variables including type, volume, concentration of the eluent, and elution time were considered in the elution step. After sorption and elution steps, the target analytes were determined by flame atomic absorption spectrometry. Limit of detection was 0.5, 0.08, 0.7, 0.4, and 0.1 ng mL−1 for Cr(III), Cu(II), Pb(II), Ni(II), and Cd(II) ions, respectively. All relative standard deviations of the method were <9.5%. The capacity of the sorbent ranged between 184 and 215 mg g−1. Finally, the developed method was successfully applied to the rapid extraction of trace amounts of these ions from black tea leaf samples and drinking water.


Similar content being viewed by others
Change history
22 November 2017
The original version of this article unfortunately contained a mistake. The sequence of the author names was incorrect.
Abbreviations
- ANOVA:
-
Analysis of variance
- BBD:
-
Box-Behnken design
- 3-CPS:
-
3-Chloropropyltriethoxysilane
- DPA:
-
Dipyridylamine
- FAAS:
-
Flame atomic absorption spectrometry
- LOD:
-
Limit of detection
- MNPs:
-
Magnetic nanoparticles
- MSPE:
-
Magnetic solid-phase extraction
- MWCNTs:
-
Multiwalled carbon nanotubes
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
References
Abe S, Fuji K, Sono T (1994) Liquid-liquid extraction of manganese(II), copper(II) and zinc(II) with acyclic and macrocyclic Schiff bases containing bisphenol A subunits. Anal Chim Acta 293:325–330
Adlnasab L, Ebrahimzadeh H, Asgharinezhad AA, Aghdam MN, Dehghani A, Esmaeilpour S (2014) A preconcentration procedure for determination of ultra-trace mercury(II) in environmental samples employing continuous-flow cold vapor atomic absorption spectrometry. Food Anal Methods 7:616–628
Asgharinezhad AA, Jalilian N, Ebrahimzadeh H, Panjali Z (2015a) A simple and fast method based on new magnetic ion imprinted polymer nanoparticles for the selective extraction of Ni(II) ions in different food samples. RSC Adv 5:45510–45519
Asgharinezhad AA, Rezvani M, Ebrahimzadeh H, Shekari N, Ahmadinasab N, Loni M (2015b) Solid phase extraction of Pb(II) and Cd(II) ions based on murexide functionalized magnetic nanoparticles with the aid of experimental design methodology. Anal Methods 7:10350–10358
Asgharinezhad AA, Ebrahimzadeh H (2015) Coextraction of acidic, basic and amphiprotic pollutants using multiwalled carbon nanotubes/magnetite nanoparticles@polypyrrole composite. J Chromatogr A 1412:1–11
Asgharinezhad AA, Ebrahimzadeh H (2016a) Poly (2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J Chromatogr A 1435:18–29
Asgharinezhad AA, Ebrahimzadeh H (2016b) Supramolecular nanosolvent-based hollow fiber liquid phase microextraction as a novel method for simultaneous preconcentration of acidic, basic and amphiprotic pollutants. RSC Adv 6:41825–41834
Asgharinezhad AA, Ebrahimzadeh H (2016c) A simple and fast method based on mixed hemimicelles coated magnetite nanoparticles for simultaneous extraction of acidic and basic pollutants. Anal Bioanal Chem 408:473–486
Asgharinezhad AA, Ebrahimzadeh H, Rezvani M, Shekari N, Loni M (2014) A novel 4-(2-pyridylazo) resorcinol functionalized magnetic nanosorbent for selective extraction of Cu (II) and Pb (II) ions from food and water samples. Food Add Contam: Part A 31:1196–1204
Bagheri H, Afkhami A, Saber-Tehrani M, Khoshsafar H (2012) Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 97:87–95
Bagheri H, Asgharinezhad AA, Ebrahimzadeh H (2016) Determination of trace amounts of Cd(II), Cu(II), and Ni(II) in food samples using a novel functionalized magnetic nanosorbent. Food Anal Methods 9:876–888
Bagtash M, Yamini Y, Tahmasebi E, Zolgharnein J, Dalirnasab Z (2016) Magnetite nanoparticles coated with tannic acid as a viable sorbent for solid-phase extraction of Cd2+, Co2+ and Cr3+. Microchim Acta 183:449–456
Bruno P, Caselli M, De Gennaro G, Ielpo P, Ladisa T, Placentino CM (2006) Ion chromatography determination of heavy metals in airborne particulate with preconcentration and large volume direct injection. Chromatographia 64:537–542
Du Z, Liu M, Li G (2013) Novel magnetic SPE method based on carbon nanotubes filled with cobalt ferrite for the analysis of organochlorine pesticides in honey and tea. J Sep Sci 36:3387–3394
Duran A, Tuzen M, Soylak M (2009) Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169:466–471
Ebrahimzadeh H, Asgharinezhad AA, Tavassoli N, Sadeghi O, Amini MM, Kamarei F (2012a) Separation and spectrophotometric determination of very low levels of Cr (VI) in water samples by novel pyridine-functionalized mesoporous silica. Int J Environ Anal Chem 92:509–521
Ebrahimzadeh H, Shekari N, Saharkhiz Z, Asgharinezhad AA (2012b) Simultaneous determination of chloropheniramine maleate and dextromethorphan hydrobromide in plasma sample by hollow fiber liquid phase microextraction and high performance liquid chromatography with the aid of chemometrics. Talanta 94:77–83
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172–177
Ghaedi M, Ahmadi F, Tavakoli Z, Montazerozohori M, Khanmohammadi A, Soylak M (2008) Three modified activated carbons by different ligands for the solid phase extraction of copper and lead. J Hazard Mater 152:1248–1255
Gürkan R, Altunay N, Yıldırım E (2016) Combination of ultrasonic-assisted cloud point extraction with flame AAS for preconcentration and determination of trace amounts of silver and cadmium in dried nut and vegetable samples. Food Anal Methods 1–12.
Hamilton JW, Kaltreider RC, Bajenova OV, Ihnat MA, McCaffrey J, Turpie BW, Rowell EE, Oh J, Nemeth MJ, Pesce CA, Lariviere JP (1998) Molecular basis for effects of carcinogenic heavy metals on inducible gene expression. Environ Health Persp 106:1005–1015
Herrero Latorre C, Alvarez Méndez J, Barciela García J, García Martín S, Peña Crecente RM (2012) Carbon nanotubes as solid-phase extraction sorbents prior to atomic spectrometric determination of metal species: a review. Anal Chim Acta 749:16–35
Kamarei F, Ebrahimzadeh H, Asgharinezhad AA (2011) Optimization of simultaneous derivatization and extraction of aliphatic amines in water samples with dispersive liquid–liquid microextraction followed by HPLC. J Sep Sci 34:2719–2725
Liu C, He S, Shen K, Feng X, Fang G, Wang S (2015) l-Cysteine functionalized silica gel as an efficient adsorbent for the determination of heavy metals in foods by ICP-MS. Food Anal Methods 8:1785
Manoochehri M, Asgharinezhad AA, Shekari N (2015) Synthesis, characterization and analytical application of Fe3O4@SiO2@polyaminoquinoline magnetic nanocomposite for the extraction and pre-concentration of Cd(II) and Pb(II) in food samples. Food Addit Contam: Part A 32:737–747
Partanen T, Heikkilä P, Hernberg S, Kauppinen T, Moneta G, Ojajärvi A (1991) Renal cell cancer and occupational exposure to chemical agents. Scand J Work Environ Health 1:231–239
Sohrabi MR, Matbouie Z, Asgharinezhad AA, Dehghani A (2013) Solid phase extraction of Cd(II) and Pb(II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim Acta 180:589–597
Soylak M, Ercan O (2009) Selective separation and preconcentration of copper (II) in environmental samples by the solid phase extraction on multi-walled carbon nanotubes. J Hazard Mater 168:1527–1531
Tadjarodi A, Abbaszadeh A (2016) A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury (II). Microchim Acta 183:1391–1399
Taghizadeh M, Asgharinezhad AA, Pooladi M, Barzin M, Abbaszadeh A, Tadjarodi A (2013) A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim Acta 180:1073–1084
Taghizadeh M, Asgharinezhad AA, Samkhaniany N, Tadjarodi A, Abbaszadeh A, Pooladi M (2014) Solid phase extraction of heavy metal ions based on a novel functionalized magnetic multi-walled carbon nanotube composite with the aid of experimental design methodology. Microchim Acta 181:597–605
Tarigh GD, Shemirani F (2013) Magnetic multi-wall carbon nanotube nanocomposite as an adsorbent for preconcentration and determination of lead (II) and manganese (II) in various matrices. Talanta 115:744–750
Tuzen M, Saygi KO, Soylak M (2008) Novel solid phase extraction procedure for gold(III) on Dowex M 4195 prior to its flame atomic absorption spectrometric determination. J Hazard Mater 156:591–595
Vallee BL, Ulmer DD (1972) Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem 41:91–128
Wadhwa SK, Tuzen M, GulKazi T, Soylak M (2013) Graphite furnace atomic absorption spectrometric detection of vanadium in water and food samples after solid phase extraction on multiwalled carbon nanotubes. Talanta 116:205–209
Wang Y, Chen H, Tang J, Ye G, Ge H, Hu X (2015) Preparation of magnetic metal organic frameworks adsorbent modified with mercapto groups for the extraction and analysis of lead in food samples by flame atomic absorption spectrometry. Food Chem 181:191–197
Xie ZH, Xie FZ, Guo LQ, Lin XC, Chen GN (2005) Thioacetamide chemically immobilized on silica gel as a solid phase extractant for the extraction and preconcentration of copper (II), lead (II), and cadmium (II). J Sep Sci 28:462–470
Yebra-Biurrun MC, Bermejo-Barrera A, Bermejo-Barrera MP, Barciela-Alonso MC (1995) Atomic absorption spectrometry determination of trace metals in natural waters by flame atomic absorption spectrometry following on-line ion-exchange preconcentration. Anal Chim Acta 303:341–345
Yilmaz AB (2003) Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay, Turkey. Environ Res 92:277–281
Acknowledgements
The authors would like to thank Central Tehran Branch, Islamic Azad University, Tehran, Iran for the financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mahboobeh Manoochehri declares that she has no conflict of interest. Leila Naghibzadeh declares that she has no conflict of interest.
Ethics Approval
This article does not contain any studies with human participants or animals performed by the authors.
Informed Consent
For this type of study, informed consent is not required.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s12161-017-1108-7.
Electronic supplementary material
.
ESM 1
(DOCX 1832 kb)
Rights and permissions
About this article
Cite this article
Manoochehri, M., Naghibzadeh, L. A Nanocomposite Based on Dipyridylamine Functionalized Magnetic Multiwalled Carbon Nanotubes for Separation and Preconcentration of Toxic Elements in Black Tea Leaves and Drinking Water. Food Anal. Methods 10, 1777–1786 (2017). https://doi.org/10.1007/s12161-016-0741-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0741-x