Abstract
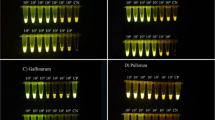
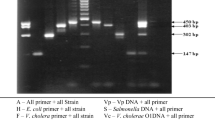
Salmonella is a major food-borne pathogen in humans and a cause of local or systemic disease in animals. Therefore, rapid and reliable methods to detect these poultry-associated Salmonella serotypes are necessary for efficient control of Salmonella in poultry. The present study aimed to develop a real-time multiplex PCR (MqPCR) method to simultaneously detect and/or differentiate Salmonella sp. and poultry-associated serotypes, including Salmonella Enteritidis, Salmonella Typhimurium, Salmonella Pullorum, and Salmonella Gallinarum. A MqPCR method was designed using four specific primer pairs and probes for the detection of Salmonella sp., including S. Enteritidis, S. Typhimurium, S. Pullorum, and S. Gallinarum. Additionally, a novel TaqMan-based MqPCR method combined with propidium monoazide (PMA) treatment was developed for the simultaneous quantification of viable cells of Salmonella sp. and these four Salmonella serotypes in rinse water of chicken carcasses. The MqPCR assay specifically detected Salmonella sp., S. Enteritidis, S. Typhimurium, S. Pullorum, and S. Gallinarum, showing 100% sensitivity and 100% specificity. This optimized PMA-MqPCR assay could detect live Salmonella (100–106 CFU/reaction) without enrichment in live/dead cell mixtures from spiked rinse water of chicken carcasses. The procedure for detecting live Salmonella required less than 2 h to complete. This PMA TaqMan-based MqPCR technique facilitates accurate and rapid monitoring of contamination with viable Salmonella. Also, the assay enables simultaneous identification of S. Enteritidis, S. Typhimurium, S. Pullorum, and S. Gallinarum in rinse water of chicken carcasses. The assay developed in this study will be useful in diagnostic laboratories for improving Salmonella control in poultry and poultry products.

Similar content being viewed by others
References
Aarestrup FM, Hendriksen RS, Lockett J, Gay K, Teates K, McDermott PF, White DG, Hasman H, Sorensen G, Bangtrakulnonth A, Pornreongwong S, Pulsrikarn C, Angulo FJ, Gerner-Smidt P (2007) International spread of multidrug-resistant Salmonella Schwarzengrund in food products. Emerg Infect Dis 13:726–731
Ahn YC, Cho MH, Yoon IK, Jung DH, Lee EY, Kim JH, Jang WC (2010) Detection of Salmonella using the loop mediated isothermal amplification and real-time PCR. J Kor Chemi Soci 54:215–221
Alizadeh AH, Behrouz N, Salmanzadeh S, Ranjbar M, Azimian MH, Habibi E, Jaafari F, Zolfagharian K, Zali MR (2007) Escherichia coli, Shigella and Salmonella species in acute diarrhoea in Hamedan, Islamic Republic of Iran. Eastern Mediterr Health J 13:243–249
Cawthorn DM, Witthuhn RC (2008) Selective PCR detection of viable Enterobacter sakazakii cells utilizing propidium monoazide or ethidium bromide monoazide. J Appl Microbiol 105:1178–1185
Cenciarini-Borde C, Courtois S, La Scola B (2009) Nucleic acids as viability markers for bacteria detection using molecular tools. Future Microbiol 4:45–64
Centers for Disease Control and Prevention (CDC) (2010) Preliminary food net data on the incidence of infection with pathogens transmitted commonly through food-10 states, 2009. MMWR Morb Mortal Wkly Rep 59:418–422 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5914a2.htm
Chen S, Wang F, Beaulieu JC, Stein RE, Ge BL (2011) Rapid detection of viable Salmonella in produce by coupling propidium monoazide with loop-mediated isothermal amplification. Appl Environ Microbiol 77:4008–4016
Chen Y, Song KY, Brown EW, Lampel KA (2010) Development of an improved protocol for the isolation and detection of Enterobacter sakazakii (Cronobacter) from powdered infant formula. J Food Prot 73:1016–1022
Cobb SP (2011) The spread of pathogens through trade in poultry meat: overview and recent developments. Rev Sci Tech 30:149–164
Contreras PJ, Urrutia H, Sossa K, Nocker A (2011) Effect of PCR amplicon length on suppressing signals from membrane-compromised cells by propidium monoazide treatment. J Microbiol Methods 87:89–95
Flekna G, Stefanic P, Wagner M, Smulders FJ, Mozina SS, Hein I (2007) Insufficient differentiation of live and dead Campylobacter jejuni and Listeria monocytogenes cells by ethidium monoazide (EMA) compromises EMA/real-time PCR. Res Microbiol 158:405–412
Guy RA, Kapoor A, Holicka J, Shepherd D, Horgen PA (2006) A rapid molecular-based assay for direct quantification of viable bacteria in slaughterhouses. J Food Prot 69:1265–1272
Hoorfar J, Ahrens P, Radström P (2000) Automated 5′ nuclease PCR assay for identification of Salmonella enterica. J Clin Microbiol 38:3429–3435
Josefsen MH, Lofstrom C, Hansen TB, Christensen LS, Olsen JE, Hoorfar J (2010) Rapid quantification of viable campylobacter bacteria on chicken carcasses, using real-time PCR and propidium monoazide treatment, as a tool for quantitative risk assessment. Appl Environ Microbiol 76:5097–5104
Josephson KL, Gerba CP, Pepper IL (1993) Polymerase chain reaction detection of nonviable bacterial pathogens. Appl Environ Microbiol 59:3513–3515
Jothikumar N, Griffiths MW (2002) Rapid detection of Escherichia coli O157:H7 with multiplex real-time PCR assays. Appl Environ Microbiol 68:3169–3171
Kobayashi H, Oethinger M, Tuohy MJ, Hall GS, Bauer TW (2009) Unsuitable distinction between viable and dead Staphylococcus aureus and Staphylococcus epidermidis by ethidium bromide monoazide. Lett Appl Microbiol 48:633–638
Kornschober C, Mikula C, Springer B (2009) Salmonellosis in Austria: situation and trends. Wien Klin Wochenschr 121:96–102
Kottwitz LBM, Back A, Leão JA, Alcocer I, Karan M, Oliveira TCRM (2008) Contaminação por Salmonella spp. em uma cadeia de produção de ovos de uma integração de postura commercial. Arq Bras Med Vet Zootec 60:496–498
Lampel KA, Chen Y (2009) Method for the isolation and detection of Enterobacter sakazakii (Cronobacter) from powdered infant formula. Int J Food Microbiol 136:179–184
Mainar-Jaime RC, Andres S, Vico JP, San RB, Garrido V, Grillo MJ (2013) Sensitivity of the ISO 6579:2002/Amd 1:2007 standard method for detection of Salmonella spp. on mesenteric lymph nodes from slaughter pigs. J Clin Microbiol 51:89–94
Malkawi HI, Gharaibeh R (2003) Multiplex PCR for the direct detection of Salmonella enterica from chicken, lamb and beef food products. J Basic Microbiol 43:328–336
Malorny B, Bunge C, Helmuth R (2007) A real-time PCR for the detection of Salmonella Enteritidis in poultry meat and consumption eggs. J Microbiol Methods 70:245–251
Malorny B, Paccassoni E, Fack P, Bunge C, Martin AP, Helmuth R (2004) Diagnostic real-time PCR for detection of Salmonella in food. Appl Environ Microbiol 70:7046–7052
Martin B, Raurich R, Garriga M, Aymerich T (2013) Effect of amplicon length in propidium monoazide quantitative PCR for the enumeration of viable cells of Salmonella in cooked ham. Food Anal Methods 6:683–690
Masters CI, Shallcross JA, Mackey BM (1994) Effect of stress treatments on the detection of Listeria monocytogenes and enterotoxigenic Escherichia coli by the polymerase chain-reaction. J Appl Bacteriol 77:73–79
Mozola MA (2006) Genetics-based methods for detection of Salmonella spp. in foods. J AOAC Int 89:517–529
Nocker A, Camper AK (2006) Selective removal of DNA from dead cells of mixed bacterial communities by use of ethidium monoazide. Appl Environ Microbiol 72:1997–2004
Nocker A, Cheung CY, Camper AK (2006) Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Methods 67:310–320
Nogva HK, Dromtrop SM, Nissen H, Rudi K (2003) Ethidium monoazide for DNA-based differentiation of viable and dead bacteria by 5′-nuclease PCR. BioTechniques 34:804–813
Rabsch W, Tschäpe H, Bäumler AJ (2001) Non-typhoidal salmonellosis: emerging problems. Microbes Infect 3:237–247
Rahn K, De Grandis SS, Clarke RC, McEwen SA, Galán JE, Ginocchio C, Curtiss R, Gyles CL (1992) Amplification of an invA gene sequence of Salmonella Typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes 6:271–279
Rossen L, Norskov P, Holmstrom K, Rasmussen OF (1992) Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int J Food Microbiol 17:37–45
Rudi K, Moen B, Dromtorp SM, Holck AL (2005) Use of ethidium monoazide and PCR in combination for quantification of viable and dead cells in complex samples. Appl Environ Microbiol 71:1018–1024
Soejima T, Schlitt-Dittrich F, Yoshida S (2011) Polymerase chain reaction amplification length-dependent ethidium monoazide suppression power for heat-killed cells of Enterobacteriaceae. Anal Biochem 418:37–43
Tatusov RL, Koonin EV, Lipman DJ (1997) A genomic perspective on protein families. Science 278:631–637
Thorns CJ (2000) Bacterial food-borne zoonoses. Rev Sci Tech 19:226–239
Wang L, Mustapha A (2010) EMA-real-time PCR as a reliable method for detection of viable Salmonella in chicken and eggs. J Food Sci 75:M134–M139
Wang S, Levin RE (2006) Discrimination of viable Vibrio vulnificus cells from dead cells in real-time PCR. J Microbiol Methods 64:1–8
Wiemer D, Loderstaedt U, von Wulffen H, Priesnitz S, Fischer M, Tannich E, Hagen RM (2011) Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. Int J Med Microbiol 301:577–584
Wilson DL, Abner SR, Newman TC, Mansfield LS, Linz JE (2000) Identification of ciprofloxacin-resistant Campylobacter jejuni by use of a fluorogenic PCR assay. J Clin Microbiol 38:3971–3978
Wilson IG (1997) Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol 63:3741–3751
Yang YG, Song MK, Park SJ, Kim SW (2007) Direct detection of Shigella flexneri and Salmonella Typhimurium in human feces by real-time PCR. J Microbiol Biotechnol 17:1616–1621
Acknowledgements
This work was supported by a grant from the QIA, Ministry of Agriculture, Food and Rural Affaires, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by QIA, Ministry of Agriculture, Food and Rural Affaires, Korea.
Conflict of Interest
So Youn Youn declares that she has no conflict of interest. Ok Mi Jeong declares that she has no conflict of interest. Byung Kook Choi declares that he has no conflict of interest. Suk Chan Jung declares that he has no conflict of interest. Min Su Kang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Publication has been approved by all individual participants.
Rights and permissions
About this article
Cite this article
Youn, S.Y., Jeong, O.M., Choi, B.K. et al. Development of a Real-Time Multiplex PCR Assay with Propidium Monoazide Treatment for Simultaneous Detection of Live Salmonella, and Salmonella Enteritidis, S. Typhimurium, S. Pullorum, and S. Gallinarum, in Rinse Water of Chicken Carcasses. Food Anal. Methods 10, 1681–1689 (2017). https://doi.org/10.1007/s12161-016-0716-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0716-y