Abstract
Objectives
We aimed to investigate the association between genetic factors of SNPs dopamine transporter (DAT) and serotonin transporter (SERT) availabilities in healthy controls.
Methods
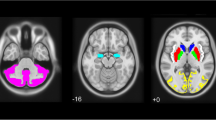
The study population consisted of healthy controls with screening 123I-FP-CIT single-photon emission computed tomography. Specific binding of 123I-FP-CIT regarding DAT and SERT was calculated using a region of interest analysis. VOI template was applied to measure specific binding ratios (SBRs) of caudate nucleus, putamen, striatum, midbrain, and pons.
Results
One hundred sixty healthy controls (male 106, female 54, 61.0 ± 11.5 years) were included in this study. Sex difference did not exist in DAT availabilities of caudate nucleus (p = 0.5344), putamen (p = 0.5006), and striatum (p = 0.5056). However, male subjects had higher SERT availabilities of both midbrain (p = 0.0436), and pons (p = 0.0061). Therefore, we analyzed the effect of SNP on DAT availabilities of subjects in all, and that on SERT availabilities of males and females separately. None of 19 SNPs included in this study showed the effect on DAT availabilities. However, rs591323 in Fibroblast Growth Factor 20 on chromosome 8 had a significant impact on SERT availability of both midbrain (p = 0.0056) and pons (p = 0.0007).
Conclusion
SNP rs591323 of risk loci for Parkinson’s disease is associated with SERT availability of healthy male subjects.


Similar content being viewed by others
References
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46(9):989–93.
Rawlik K, Rowlatt A, Tenesa A. Imputation of DNA methylation levels in the brain implicates a risk factor for Parkinson’s disease. Genetics. 2016;204(2):771–81.
Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, Buscombe J. The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 1. AJNR Am J Neuroradiol. 2015;36(2):229–35.
Zipursky RB, Meyer JH, Verhoeff NP. PET and SPECT imaging in psychiatric disorders. Can J Psychiatry. 2007;52(3):146–57.
Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord. 2003;18(12):1415–23.
Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41(10):1938–46.
Joutsa J, Johansson J, Seppanen M, Noponen T, Kaasinen V. Dorsal-to-ventral shift in midbrain dopaminergic projections and increased thalamic/raphe serotonergic function in early Parkinson disease. J Nucl Med Off Publ Soc Nucl Med. 2015;56(7):1036–41.
Booij J, de Jong J, de Bruin K, Knol R, de Win MM, van Eck-Smit BL. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med Off Publ Soc Nucl Med. 2007;48(3):359–66.
Roselli F, Pisciotta NM, Pennelli M, Aniello MS, Gigante A, De Caro MF, et al. Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord. 2010;25(12):1853–9.
van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50(1):45–52.
Parkinson Progression Marker I. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629–35.
Garcia-Gomez FJ, Garcia-Solis D, Luis-Simon FJ, Marin-Oyaga VA, Carrillo F, Mir P, et al. Elaboration of the SPM template for the standardization of SPECT images with 123I-ioflupane. Rev Esp Med Nucl Imagen Mol. 2013;32(6):350–6.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89.
Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, et al. Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med Off Publ Soc Nucl Med. 2000;41(1):36–44.
van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, et al. Age-related decline in central serotonin transporter availability with [(123)I]beta-CIT SPECT. Neurobiol Aging. 2000;21(4):497–501.
Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27(7):867–9.
France M, Skorich E, Kadrofske M, Swain GM, Galligan JJ. Sex-related differences in small intestinal transit and serotonin dynamics in high-fat-diet-induced obesity in mice. Exp Physiol. 2016;101(1):81–99.
Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology. 2014;231(8):1581–99.
Redensek S, Trost M, Dolzan V. Genetic determinants of Parkinson’s disease: can they help to stratify the patients based on the underlying molecular defect? Front Aging Neurosci. 2017;9:20.
Contin M, Martinelli P, Mochi M, Albani F, Riva R, Scaglione C, et al. Dopamine transporter gene polymorphism, spect imaging, and levodopa response in patients with Parkinson disease. Clin Neuropharmacol. 2004;27(3):111–5.
Muellner J, Gharrad I, Habert MO, Kas A, Martini JB, Cormier-Dequaire F, et al. Dopaminergic denervation severity depends on COMT Val158Met polymorphism in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(5):471–6.
Sun XY, Wang L, Cheng L, Li NN, Lu ZJ, Li JY, et al. Genetic analysis of FGF20 in Chinese patients with Parkinson’s disease. Neurol Sci. 2017;38(5):887–91.
Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, et al. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82(2):283–9.
van der Walt JM, Noureddine MA, Kittappa R, Hauser MA, Scott WK, McKay R, et al. Fibroblast growth factor 20 polymorphisms and haplotypes strongly influence risk of Parkinson disease. Am J Hum Genet. 2004;74(6):1121–7.
Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J. Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Mol Psychiatry. 2014;19(8):880–9.
Willeit M, Praschak-Rieder N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: A review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. Neuroimage. 2010;53(3):878–92.
Chen PS, Yeh TL, Lee IH, Lin CB, Tsai HC, Chen KC, et al. Effects of C825T polymorphism of the GNB3 gene on availability of dopamine transporter in healthy volunteers–a SPECT study. Neuroimage. 2011;56(3):1526–30.
Chang WH, Lee IH, Chen KC, Chi MH, Chiu NT, Yao WJ, et al. Oxytocin receptor gene rs53576 polymorphism modulates oxytocin–dopamine interaction and neuroticism traits—a SPECT study. Psychoneuroendocrinology. 2014;47:212–20.
Acknowledgements
PPMI—a public–private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including abbVie, Avid, Biogen, Bristol-Myers Squibb, COVANCE, GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Lilly, Merck, MesoScaleDiscovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, TEVA, and UCB.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pak, K., Nam, HY., Shin, S. et al. Effects of rs591323 on serotonin transporter availability in healthy male subjects. Ann Nucl Med 32, 431–436 (2018). https://doi.org/10.1007/s12149-018-1262-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1262-z