Abstract
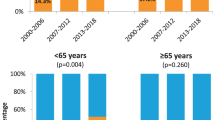
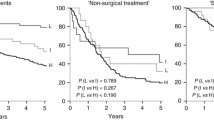
Human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) is related to improved treatment outcomes. What remains unclear is whether all HPV DNA genotypes carry similar prognostic relevance. We aimed to evaluate disease control and survival outcomes by HPV DNA genotype. Patients with primary OPSCC without distant metastases treated with curative intent were retrospectively identified from an IRB-approved institutional database. Patients that underwent HPV DNA polymerase chain reaction (PCR) testing with available genotype were included and dichotomized by the presence of HPV type 16 (HPV-16) or other high-risk HPV genotype (HPV-non16). Overall survival (OS), disease-free survival (DFS), locoregional control (LRC) and distant control (DC) were determined using the Kaplan–Meier method and compared using the log-rank test. In our cohort of 193 patients treated from 2012 to 2018 with HPV DNA PCR, 10% were detected as HPV-non16 high-risk types. Patients with HPV-16 were significantly younger than those with HPV-non16, but no other baseline factors were associated with HPV-non16. With a median follow-up of 42.9 months, there were no significant differences in outcomes between the HPV-16 and HPV-non16 groups for 3-year OS (87.7% v. 73.6%), DFS (82.9% v. 68.7%), LRC (92.8% v. 88.5%) or DC (91% v. 89.2%). There is no statistically significant difference in outcomes between OPSCC with HPV-16 and HPV-non16 high-risk genotypes in our cohort, though trends of overall worse survival and disease-free survival in HPV-non 16 OPSCC were seen. Further studies with larger cohorts of patients with HPV-non 16-associated OPSCC are required to make definitive conclusions regarding the prognostic and clinical significance of HPV type.


Similar content being viewed by others
References
Gillison ML. Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination. J Adolesc Health. 2008;43(4 Suppl):S52-60.
Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–60.
Dayyani F, et al. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15.
Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48-54.
Gillison ML, et al. Eurogin Roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer. 2014;134(3):497–507.
Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Mallen-St Clair J, et al. Human papillomavirus in oropharyngeal cancer: the changing face of a disease. Biochim Biophys Acta. 2016;1866(2):141–50.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al, editors. AJCC cancer staging manual. 8th ed. Springer International Publishing, American Joint Commission on Cancer; 2017.
Chera BS, Amdur RJ. Current status and future directions of treatment deintensification in human papilloma virus-associated oropharyngeal squamous cell carcinoma. Semin Radiat Oncol. 2018;28(1):27–34.
Keck MK, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–81.
Grce M, Mravak-Stipetic M. Human papillomavirus-associated diseases. Clin Dermatol. 2014;32(2):253–8.
de Villiers EM, Gissmann L, Zur Hausen H. Molecular cloning of viral DNA from human genital warts. J Virol. 1981;40(3):932–5.
Munger K, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60.
Harris PA, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Nagatsuka M, et al. Omitting elective irradiation of the contralateral retropharyngeal nodes in oropharyngeal squamous cell carcinoma treated with intensity-modulated radiotherapy. Cureus. 2019;11(1):e3825.
Elrefaey S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299–309.
Varier I, et al. Clinical characteristics and outcomes of oropharyngeal carcinoma related to high-risk non-human papillomavirus16 viral subtypes. Head Neck. 2016;38(9):1330–7.
St Guily JL, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France-The EDiTH VI study. J Clin Virol. 2011;51(2):100–4.
Chaturvedi AK, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9.
Misiukiewicz K, et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: the quarterback trial. Oral Oncol. 2019;95:170–7.
Weinberger PM, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–47.
Semrau R, et al. Prognostic impact of human papillomavirus status, survivin, and epidermal growth factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head Neck. 2013;35(9):1339–44.
Driessen CM, et al. Toxicity and efficacy of accelerated radiotherapy with concurrent weekly cisplatin for locally advanced head and neck carcinoma. Head Neck. 2016;38(Suppl 1):E559–65.
Lewis JS Jr, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the college of American pathologists. Arch Pathol Lab Med. 2018;142(5):559–97.
El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–45.
Westra WH. The pathology of HPV-related head and neck cancer: implications for the diagnostic pathologist. Semin Diagn Pathol. 2015;32(1):42–53.
Fonmarty D, et al. Study of the concordance between p16 immunohistochemistry and HPV-PCR genotyping for the viral diagnosis of oropharyngeal squamous cell carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(3):135–9.
Pannone G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 immunohistochemistry, consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer. 2012;7:4.
Albers AE, et al. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci Rep. 2017;7(1):16715.
Liu JC, et al. High prevalence of discordant human papillomavirus and p16 oropharyngeal squamous cell carcinomas in an African American cohort. Head Neck. 2016;38(Suppl 1):E867–72.
Froberg M, et al. Impact of the human papillomavirus status on the development of high-grade cervical intraepithelial neoplasia in women negative for intraepithelial lesions or malignancy at the baseline: a 9-year Swedish nested case-control follow-up study. Cancer. 2019;125(2):239–48.
Hopenhayn C, et al. Prevalence of human papillomavirus types in invasive cervical cancers from 7 US cancer registries before vaccine introduction. J Low Genit Tract Dis. 2014;18(2):182–9.
Funding
This work was supported by the Wake Forest Baptist Medical Center and National Center for Advancing Translational Sciences (NCATS), National Institutes of Health funded Wake Forest Clinical and Translational Science Institute (WF CTSI) through Grant Award Number UL1TR001420.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to be declared.
Ethical Approval
This study was reviewed by the Wake Forest Clinical and Translational Science Institute IRB and approved by ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shenker, R.F., May, N.H., Waltonen, J.D. et al. Comparing Outcomes for Patients with Human Papillomavirus (HPV) Type 16 versus Other High-Risk HPV Types in Oropharyngeal Squamous Cell Carcinoma. Head and Neck Pathol 15, 866–874 (2021). https://doi.org/10.1007/s12105-021-01308-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01308-6