Abstract
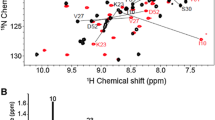
FROUNT is a cytoplasmic protein that interacts with the membrane-proximal C-terminal regions (Pro-Cs) of the CCR2 and CCR5 chemokine receptors. The interactions between FROUNT and the chemokine receptors play an important role in the migration of inflammatory immune cells. Therefore, FROUNT is a potential drug target for inflammatory diseases. However, the structural basis of the interactions between FROUNT and the chemokine receptors remains to be elucidated. We previously identified the C-terminal region (residues 532–656) of FROUNT as the structural domain responsible for the Pro-C binding, referred to as the chemokine receptor-binding domain (CRBD), and then constructed its mutant, bearing L538E/P612S mutations, with improved NMR spectral quality, referred to as CRBD_LEPS. We now report the main-chain and side-chain 1H, 13C, and 15N resonance assignments of CRBD_LEPS. The NMR signals of CRBD_LEPS were well dispersed and their intensities were uniform on the 1H–15N HSQC spectrum, and thus almost all of the main-chain and side-chain resonances were assigned. This assignment information provides the foundation for NMR studies of the three-dimensional structure of CRBD_LEPS in solution and its interactions with chemokine receptors.

Similar content being viewed by others
References
Cavanagh J, Fairbrother W, Palmer A III, Skelton N, Rance M (2007) Protein NMR spectroscopy: principles and practice, 2nd edn. Elsevier Academic Press, San Diego
Delaglio F, Grzesiek S, Vuister G, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Esaki K, Terashima Y, Toda E, Yoshinaga S, Araki N, Matsushima K, Terasawa H (2011) Expression and purification of human FROUNT, a common cytosolic regulator of CCR2 and CCR5. Protein Expr Purif 77:86–91
Esaki K, Yoshinaga S, Tsuji T, Toda E, Terashima Y, Saitoh T, Kohda D, Kohno T, Osawa M, Ueda T, Shimada I, Matsushima K, Terasawa H (2014) Structural basis for the binding of the membrane-proximal C-terminal region of chemokine receptor CCR2 with the cytosolic regulator FROUNT. FEBS J 281:5552–5566
Marsh JA, Singh VK, Jia Z, Forman-Kay JD (2006) Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci 15:2795–2804
Sonoda A, Yoshinaga S, Yunoki K, Ezaki S, Yano K, Takeda M, Toda E, Terashima Y, Matsushima K, Terasawa H (2017) Identification and preparation of a novel chemokine receptor-binding domain in the cytoplasmic regulator FROUNT. Mol Biotechnol 59:141–150
Terashima Y, Onai N, Murai M, Enomoto M, Poonpiriya V, Hamada T, Motomura K, Suwa M, Ezaki T, Haga T, Kanegasaki S, Matsushima K (2005) Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat Immunol 6:827–835
Toda E, Terashima Y, Sato T, Hirose K, Kanegasaki S, Matsushima K (2009) FROUNT is a common regulator of CCR2 and CCR5 signaling to control directional migration. J Immunol 183:6387–6394
Toda E, Terashima Y, Esaki K, Yoshinaga S, Sugihara M, Kofuku Y, Shimada I, Suwa M, Kanegasaki S, Terasawa H, Matsushima K (2014) Identification of a binding element for the cytoplasmic regulator FROUNT in the membrane-proximal C-terminal region of chemokine receptors CCR2 and CCR5. Biochem J 457:313–322
Yunoki K, Yoshinaga S, Takeda M, Nagano R, Tsuchiya Y, Sonoda A, Tsuji T, Hirakane M, Toda E, Terashima Y, Matsushima K, Terasawa H (2018) Efficient identification of compounds suppressing protein precipitation via solvent screening using serial deletion mutants of the target protein. Genes Cells 23:70–79
Acknowledgements
We gratefully acknowledge M. Yokochi and F. Inagaki for NMR analyses. This work was supported in part by the Targeted Proteins Research Program (TPRP) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (to S.Y., E.T., Y.T., K.M. and H.T.), by Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED) (to S.Y., M.T., E.T., Y.T., K.M. and H.T.), by Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT) from AMED (to K.M.), by Project for Cancer Research and Therapeutic Evolution (P-CREATE) from AMED (to S.Y., M.T., Y.T., K.M. and H.T.), by a Grant-in-Aid for Young Scientists (B) (JP19790064) from MEXT (to S.Y.), by the KUMAYAKU Alumni Research Fund (to S.Y.), and by the Adaptable and Seamless Technology Transfer Program through target-driven R&D (A-STEP), Japan Science and Technology Agency (JST) (to H.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical approval
The experiments described in this paper comply with the current law of Japan.
Rights and permissions
About this article
Cite this article
Yoshinaga, S., Ishida, N., Tsuji, T. et al. 1H, 13C and 15N resonance assignments for a chemokine receptor-binding domain of FROUNT, a cytoplasmic regulator of chemotaxis. Biomol NMR Assign 12, 259–262 (2018). https://doi.org/10.1007/s12104-018-9819-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-018-9819-2