Abstract
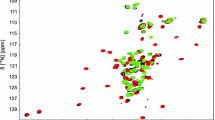
The gram-negative organism Pseudomonas aeruginosa is an opportunistic human pathogen and a leading cause of hospital-acquired infections. In P. aeruginosa PAO1, three cytoplasmic thioredoxins have been identified. An unusual thioredoxin (Patrx2) (108 amino acids) encoded by the PA2694 gene, is identified as a new thioredoxin-like protein based on sequence homology. Thioredoxin is a ubiquitous protein, which serves as a general protein disulfide oxidoreductase. Patrx2 present an atypical active site CGHC. We report the nearly complete 1H, 13C and 15N resonance assignments of reduced Patrx2. 2D and 3D heteronuclear NMR experiments were performed with uniformly 15N-, 13C-labelled Patrx2, resulting in 97.2 % backbone and 92.5 % side-chain 1H, 13C and 15N resonance assignments for the reduced form. (BMRB deposits with accession number 18130).



Similar content being viewed by others
References
Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y (2006) Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50:43–48
Chivers PT, Laboissière MC, Raines RT (1996) The CXXC motif: imperatives for the formation of native disulfide bonds in the cell. EMBO J 15:2659–2667
Gouet P, Courcelle E, Stuart DI, Métoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15:305–308
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54:237–271
Keller R (2004) The computer aided resonance assignment tutorial. Cantina Verlag
Martin JL (1995) Thioredoxin—a fold for all reasons. Structure 3:245–250
Quan S, Schneider I, Pan J, Von Hacht A, Bardwell JCA (2007) The CXXC motif is more than a redox rheostat. J Biol Chem 282:28823–28833
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223
Shouldice SR, Heras B, Jarrott R, Sharma P, Scanlon MJ, Martin JL (2010) Characterization of the DsbA oxidative folding catalyst from Pseudomonas aeruginosa reveals a highly oxidizing protein that binds small molecules. Antioxid Redox Signal 12:921–931
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcin, E.B., Bornet, O., Elantak, L. et al. 1H, 13C and 15N backbone and side-chain chemical shift assignments for reduced unusual thioredoxin Patrx2 of Pseudomonas aeruginosa . Biomol NMR Assign 8, 247–250 (2014). https://doi.org/10.1007/s12104-013-9493-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-013-9493-3