Abstract
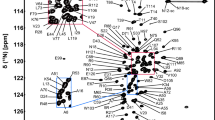
Thioredoxins are ubiquitous key antioxidant enzymes which play an essential role in cell defense against oxidative stress. They maintain the redox homeostasis owing to the regulation of thiol-disulfide exchange. In the present paper, we report the full resonance assignments of 1H, 13C and 15N atoms for the reduced and oxidized forms of Desulfovibrio vulgaris Hildenborough thioredoxin 1 (Trx1). 2D and 3D heteronuclear NMR experiments were performed using uniformly 15N-, 13C-labelled Trx1. Chemical shifts of 97% of the backbone and 90% of the side chain atoms were obtained for the oxidized and reduced form (BMRB deposits with accession number 17299 and 17300, respectively).


Similar content being viewed by others
References
Arnér ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267:6102–6109
Grzesiek S, Bax A, Clore GM, Gronenborn AM, Hu JS, Kaufman J, Palmer I, Stahl SJ, Wingfield PT (1996) The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol 3:340–345
Huber HE, Russel M, Model P, Richardson CC (1986) Interaction of mutant thioredoxins of Escherichia coli with the gene 5 protein of phage T7. The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J Biol Chem 261:15006–15012
Keller R (2004) Computer aided resonance assignment tutorial
Martin JL (1995) Thioredoxin–a fold for all reasons. Structure 3:245–250
Powis G, Oblong JE, Gasdaska PY, Berggren M, Hill SR, Kirkpatrick DL (1994) The thioredoxin/thioredoxin reductase redox system and control of cell growth. Oncol Res 6:539–544
Wolosiuk RA, Ballicora MA, Hagelin K (1993) The reductive pentose phosphate cycle for photosynthetic CO2 assimilation: enzyme modulation. FASEB J 7:622–637
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcin, E.B., Bornet, O., Pieulle, L. et al. 1H, 13C and 15N chemical shift assignments of the thioredoxin from the obligate anaerobe Desulfovibrio vulgaris Hildenborough. Biomol NMR Assign 5, 177–179 (2011). https://doi.org/10.1007/s12104-011-9294-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-011-9294-5