Abstract
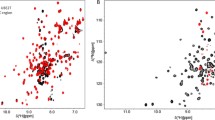
To facilitate NMR spectroscopy studies of interactions with various ligands and potential inhibitors, we report the NMR backbone resonance assignments for the 26 kD human enzyme UCH-L3, a member of the ubiquitin C-hydrolase family of ubiquitin-specific cysteine proteases.

Similar content being viewed by others
References
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfiefer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Kraulis PJ (1989) ANSIG - a program for the assignment of protein 1H 2D-NMR spectra by interactive computer-graphics. J Magn Reson 24:627–633
Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP (1997) Crystal structure of a deubiquitinating enzyme (human UCH-L3) at 1.8 A resolution. EMBO J 16:3787–3796
Misaghi S, Galardy PJ, Meester WJN, Ovaa H, Ploegh HL, Gaudet R (2005) Structure of the ubiquitin hydrolase UCH-L3 complexed with a suicide substrate. J Biol Chem 280:1512–1520
Miyoshi Y, Nakayama S, Torikoshi Y, Tanaka S, Ishihara H, Taguchi T, Tamaki Y, Noguchi S (2006) High expression of ubiquitin carboxy-terminal hydrolase-L1 and -L3 mRNA predicts early recurrence in patients with invasive breast cancer. Cancer Sci 97:523–529
Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R (2006) A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Yamazaki T, Lee W, Arrowsmith CH, Muhandiram DR, Kay LE (1994) A suite of triple-resonance NMR experiments for the backbone assignment of 15N, 13C, 2H labeled proteins with high-sensitivity. J Am Chem Soc 116:11655–11666
Acknowledgements
We acknowledge Dr Beatriz Simas Magalhaes for fruitful discussions of aspects of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harris, R., Eidhoff, U., Vinzenz, D. et al. Backbone 1H, 13C, and 15N resonance assignments for the 26-kD human de-ubiquitinating enzyme UCH-L3. Biomol NMR Assign 1, 51–53 (2007). https://doi.org/10.1007/s12104-007-9012-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-007-9012-5