Abstract
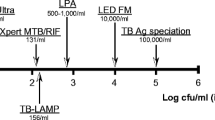
Childhood tuberculosis does not get the attention it deserves, both in the general child health services and the TB specific services. The difficulty in identification of the organism due to lack of proper sample as well as lower sensitivity of the smear, made it harder to detect cases with ease in the community. Newer diagnostic methods like cartridge based nucleic acid amplification tests (CBNAAT) and line probe assays (LPA) have the capacity to rapidly identify Mycobacterium tuberculosis with an improved sensitivity over the smear testing and have been employed under Revised National Tuberculosis Control Programme (RNTCP) across the country. As the symptoms suggestive of TB are very common and overlapping, the final yield of TB testing is better if the microbiological confirmation is done on good quality specimen from cases suspected of TB based on clinical and radiological abnormalities. Newer tests also provide simultaneous detection of much critical Rifampicin resistance. A Rifampicin resistant case is not only unlikely to respond to first-line standard therapy but such a treatment can result in further amplification of resistance to other companion drugs. Prevention of spread of the drug resistant disease thus requires that the treatment is guided by universal drug sensitivity testing (U-DST) of all TB cases. Furthermore, several changes have come up in the treatment of TB and are discussed. The dosages of anti TB drugs have been revised upwardly for optimal drug levels and now the fixed drug combinations are used under RNTCP. With the awareness about high initial Isoniazid (INH) resistance and its contribution to failure of retreatment regime, a companion third drug (Ethambutol) has been added to the continuation phase of the first-line therapy. The standard retreatment regime, better known as category II therapy, has been replaced by specific therapy as per the resistance pattern detected. The TB control activities have thus evolved a lot and the present article discusses the evolution and the current status of diagnostics and therapy of TB in children.
Similar content being viewed by others
References
WHO. Roadmap towards ending TB in children and adolescents; 2018. Available at: https://www.who.int/tb/publications/2018/tb-childhoodroadmap/en/. Accessed 20th May 2019.
Management of Pediatric Tuberculosis under the Revised National Tuberculosis Control Program (RNTCP). A Joint Statement of the Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, and Experts from Indian Academy of Pediatrics. Indian Pediatr. 2004;41:901–5.
Bates M, O'Grady J, Maeurer M, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect Dis. 2013;13:36–42.
Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84.
Marais BJ, Obihara CC, Gie RP, et al. The prevalence of symptoms associated with pulmonary tuberculosis in randomly selected children from a high burden community. Arch Dis Child. 2005;90:1166–70.
Singh S, Singh A, Prajapati S, et al; Delhi Pediatric TB Study Group. Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiol. 2015;15:191.
Lawn SD, Zumla AI. Diagnosis of extrapulmonary tuberculosis using the Xpert® MTB/RIF assay. Exp Rev Anti-Infect Ther. 2012;10:631–5.
World Health Organization. The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs: policy guidance. 2016. Available at: http://www.who.int/iris/handle/10665/246131. Accessed 20th May 2019.
McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of south African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48:1547–53.
Thee S, Seddon JA, Donald PR, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55:5560–7.
Ramachandran G, Kumar AK, Swaminathan S. Pharmacokinetics of anti-tuberculosis drugs in children. Indian J Pediatr. 2011;78:435–42.
Mukherjee A, Velpandian T, Singla M, Kanhiya K, Kabra SK, Lodha R. Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in HIV-infected Indian children. Int J Tuberc Lung Dis. 2016;20:666–72.
Graham SM. Treatment of paediatric TB: revised WHO guidelines. Paediatr Respir Rev. 2011;12:22–6.
WHO, UNICEF Statement on the Use of Child-Friendly Fixed-Dose Combinations for the Treatment of TB in Children; 2017. Available at: https://www.who.int/tb/FDC_Factsheet.pdf. Accessed 20 May 2019
Standards for TB Care in India: Ministry of Health and Family Welfare, Government of India and WHO SEARO; 2014. Available at: https://tbcindia.gov.in/showfile.php?lid=3061. Accessed 20th May 2019.
Menzies D, Benedetti A, Paydar A, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000150.
Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:223–4.
Munje R, Deshmukh R, Tumane K. Multidrug-resistant TB among previously treated TB cases: a retrospective study in Nagpur, India. Indian J Tuberc. 2015;62:207–10.
Deepa D, Achanta S, Jaju J, et al. The impact of isoniazid resistance on the treatment outcomes of smear positive re-treatment tuberculosis patients in the state of Andhra Pradesh, India. PLoS One. 2013;8:e76189.
Srinath S, Sharath B, Santosha K, et al. Tuberculosis 'retreatment others': profile and treatment outcomes in the state of Andhra Pradesh, India. Int J Tuberc Lung Dis. 2011;15:105–9.
WHO Treatment Guidelines for Isoniazid Resistant Tuberculosis: Supplement to the WHO Treatment Guidelines for Drug-Resistant Tuberculosis. Available at: https://www.who.int/tb/publications/2018/WHO_guidelines_isoniazid_resistant_TB/en/. Accessed 20 May 2019
Report of First National Anti-TB Drug Resistance Survey of India (2014–16). Ministry of Health and Family Welfare, Government of India. Available at: https://tbcindia.gov.in/showfile.php?lid=3315. Accessed 20th May 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, V. Pediatric TB Management under RNTCP: What and Why?. Indian J Pediatr 86, 707–713 (2019). https://doi.org/10.1007/s12098-019-03001-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-03001-7