Abstract
Objective
To identify predictors of complicated parapneumonic effusion (CPE)/empyema in patients of community acquired pneumonia (CAP) by using clinical and simple laboratory variables like hemoglobin (Hb), serum C-reactive protein (CRP), serum albumin (SA) levels and total leukocyte counts (TLC).
Methods
This prospective case-control study was conducted after institutional ethical approval. Subjects between ages of 2–59 mo with World Health Organization (WHO) defined CAP with written, informed parental consent were included. Cases had CAP with CPE/empyema diagnosed by pleurocentesis. Controls had severe CAP without significant pleural collection on chest X-ray (CXR). Patients with congenital and chronic diseases/infections and possible immune deficiency were excluded. Variables with univariate association with case-control status were considered as potential predictors. Final prediction model was developed by Forward Stepwise Logistic Regression (FSLR). Adjusted odd’s ratios (Adj OR) were smoothened into nearest whole numbers to develop KGMU-CPE score.
Results
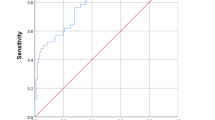
From 2016 to 17, 30 cases (66.6% males, age 38.7 + 14.9 mo) and 118 controls (78% males, age 17.8 + 16.9 mo) were included. In FSLR, predictors of CPE/empyema were ibuprofen intake (adj OR 6.8; 95%CI: 1.07–43.6), infective focus elsewhere (adj OR 28.2; 95%CI: 1.4–563.1), hypoalbuminemia <3.1 g/dL (adj OR 6.9; 95%CI: 1.22–39.3), serum CRP >20 mg/dL (adj OR 59; 95%CI: 1.86–1874.7), Hb <10 g/dL (adj OR 21.1; 95%CI: 2.8–158.1) and TLC >10,000 (adj OR 37; 95%CI: 5.7–239.8) and these six variables formed KGMU-CPE Score with a minimum score of 0 and maximum of 25. KGMU-CPE score area under the ROC curve was 0.97 and cut- off 15.55 had sensitivity of 80% and specificity of 94% for predicting CPE/empyema.
Conclusions
Using simple clinical and laboratory parameters it is possible to predict CAP with CPE/empyema. Use of ibuprofen is to be avoided in CAP as it associated with CPE. KGMU-CPE score had good diagnostic accuracy and needs external validation.


Similar content being viewed by others
References
World Health Organization (WHO) Pneumonia Fact Sheet. Updated September 2016, Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed on 26th June 2018.
Krenke K, Krawiec M, Kraj G, Peradzynska J, Krauze A, Kulus M. Risk factors for local complications in children with community-acquired pneumonia. Clin Respir J. 2018;12:253–61.
Maziah W, Choo KE, Ray JG, Ariffin WW. Empyema thoracis in hospitalized children in Kelantan, Malaysia. J Trop Pediatr. 1995;41:185–8.
Mishra OP, Das BK, Jain AK, Lahiri TK, Sen PC, Bhargara V. Clinico-bacteriological study of empyema thoracis in children. J Trop Pediatr. 1993;39:380–1.
Deiros Bronte L, Baquero-Artigao F, García-Miguel MJ, Hernández González N, Peña García P, del Castillo Martín F. Parapneumonic pleural effusion: an 11-year review. An Pediatr (Barc). 2006;64:40–5.
Anstadt MP, Guill CK, Ferguson ER, et al. Surgical versus nonsurgical treatment of empyema thoracis: an outcomes analysis. Am J Med Sci. 2003;326:9–14.
Dass R, Deka NM, Barman H, et al. Empyema thoracis: analysis of 150 cases from a tertiary care Centre in north East India. Indian J Pediatr. 2011;78:1371–7.
Strange C, Tomlinson JR, Wilson C, Harley R, Miller KS, Sahn SA. The histology of experimental pleural injury with tetracycline, empyema, and carrageenan. Exp Mol Pathol. 1989;51:205–19.
Givan DC, Eigen H. Common pleural effusions in children. Clin Chest Med. 1998;19:363–71.
World Health Organization. Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries. Available at: http://apps.who.int/iris/bitstream/10665/137319/1/9789241507813_eng.pdf. Accessed on 26th June 2018.
Balfour-Lynn IM, Abrahamson E, Cohen G, et al. BTS guidelines for the management of pleural infection in children. Thorax. 2005;60:i1–21.
FAO, WHO. World Declaration and Plan of Action for Nutrition. International Conference on Nutrition. Rome, Food and Agriculture Organization of the United Nations, December 1992. Available at: http://whqlibdoc.who.int/hq/1992/a34303.pdf. Accessed on 26th June 2018.
Prais D, Kuzmenko E, Amir J, Harel L. Association of hypoalbuminemia with the presence and size of pleural effusion in children with pneumonia. Pediatrics. 2008;121:e533–8.
Niemi E, Korppi M. Parapneumonic empyema in children before the era of pneumococcal vaccination. Acta Paediatr. 2011;100:1230–3.
World Health Organization. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Available at: http://apps.who.int/iris/bitstream/10665/66956/1/WHO_V_and_B_01.35.pdf. Accessed on 26th June 2018.
WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006.
Prober CG, Srinivas NS, Mathew R. Central nervous system infections. In: Kleigman, Staton, St Geme, et al editors. Nelson Text book of Pediatrics, 20th ed. Philadelphia: Elsevier; 2016. p. 2936–46.
Raosoft Sample size Calculator. Available at: http://www.raosoft.com/samplesize.html. Accessed on 2nd August 2016.
Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;118:1158–71.
François P, Desrumaux A, Cans C, Pin I, Pavese P, Labarère J. Prevalence and risk factors of suppurative complications in children with pneumonia. Acta Paediatr. 2010;99:861–6.
Le Bourgeois M, Ferroni A, Leruez-Ville M, et al. Nonsteroidal anti-inflammatory drug without antibiotics for acute viral infection increases the empyema risk in children: a matched case-control study. J Pediatr. 2016;175:47–53.
Mazaleuskaya LL, Theken KN, Gong L, et al. PharmGKB summary: ibuprofen pathways. Pharmacogenet Genomics. 2015;25:96–106.
Stevens DL. Could nonsteroidal anti-inflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21:977–80.
Solberg CO. Influence of phenylbutazone on the phagocytic and bactericidal activities of neutrophil granulocytes. Acta Pathol Microbiol Scand B: Microbiol Immunol. 1974;82:258–62.
Factor SH, Levine OS, Harrison LH, et al. Risk factors for pediatric invasive group a streptococcal disease. Emerg Infect Dis. 2005;11:1062–6.
Dilber E, Cakir M, Kalyoncu M, Okten A. C-reactive protein: a sensitive marker in the management of treatment response in parapneumonic empyema of children. Turkish J Pediatr. 2003;45:311–4.
Hsieh YC, Hsueh PR, Chun-Yi L, Lee PI, Lee CY, Huang LM. Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by Streptococcus pneumoniae in children in Taiwan. Clin Infect Dis. 2004;38:830–5.
Izhakian S, Wasser WG, Fox BD, Vainshelboim B, Kramer MR. The diagnostic value of the pleural fluid C-reactive protein in parapneumonic effusions. Dis Markers. 2016;2016:7539780.
Wexler ID, Knoll S, Picard E, et al. Clinical characteristics and outcome of complicated pneumococcal pneumonia in a pediatric population. Pediatr Pulmonol. 2006;41:726–34.
Kopec JA, Esdaile JM. Bias in case-control studies. A review. J Epidemiol Community Health. 1990;44:179–86.
Laupacis A, Sekar M, Stiell IG. Clinical prediction rules: a review and suggested modifications of methodological standards. JAMA. 1997;277:488–94.
Childs JD, Cleland JA. Development and application of clinical prediction rules to improve decision-making in physical therapist practice. Phys Ther. 2006;86:122–31.
Author information
Authors and Affiliations
Contributions
SA: Conceptualization. Both authors are responsible for data collection, analysis and writing the manuscript. This was MD thesis work of Meganathan P. SA will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
P, M., Awasthi, S. Predicting Complicated Parapneumonic Effusion in Community Acquired Pneumonia: Hospital Based Case-Control Study. Indian J Pediatr 86, 140–147 (2019). https://doi.org/10.1007/s12098-018-2769-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-018-2769-y